LAWSUITS NEWS & LEGAL INFORMATION
ReNu Contact Lens Solution Recall
In April, 2006 Bausch & Lomb--the makers of Renu with MoistureLoc--issued a worldwide recall after its contact lens solution was linked to hundreds of cases of Fusarium Keratitis, a rare eye infection that can cause severe eyesight damage and even blindness.
Researchers with the Center for Medical Mycology (CMM) found that the strain of Fusarium fungus responsible for the Renu outbreak had the ability to form biofilms. One of CMM's main areas of focus is devoted to fungal biofilms--a mix of bacteria and sticky extracellular material that made the Fusarium extremely resistant to contact lens solutions and the body's own immune system. Lesser symptoms of fungal eye infection include blurred vision, eye pain, eye redness, sensitivity to light and discharge.
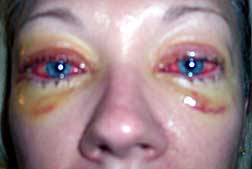 In a warning letter back in October, 2006 the FDA criticized Bausch & Lomb for neglecting to report nearly 36 eye infections linked to Renu that occurred before sales of the product were suspended. The letter also cited the company for numerous violations at its Greenville, South Carolina manufacturing plant.
In a warning letter back in October, 2006 the FDA criticized Bausch & Lomb for neglecting to report nearly 36 eye infections linked to Renu that occurred before sales of the product were suspended. The letter also cited the company for numerous violations at its Greenville, South Carolina manufacturing plant.
(In March 2007, Bausch & Lomb issued a voluntary recall of another contact lens product, its Renu Multiplus Solution--also manufactured at the same plant--this time for having trace amounts of iron, which could apparently lead to a shortened shelf life for the solution.)
 Bausch & Lomb now faces at least 400 consumer product liability lawsuits in the US from victims claiming injury from Renu, and it also faces lawsuits from shareholders. One suit alleges that the product was "unreasonably dangerous and defective" due to contamination and that Bausch & Lomb failed to warn consumers of the dangers associated with the product. The plaintiff suffered extreme pain and anguish and was hospitalized. She now faces additional upcoming surgeries to possibly remove her eye.
Bausch & Lomb now faces at least 400 consumer product liability lawsuits in the US from victims claiming injury from Renu, and it also faces lawsuits from shareholders. One suit alleges that the product was "unreasonably dangerous and defective" due to contamination and that Bausch & Lomb failed to warn consumers of the dangers associated with the product. The plaintiff suffered extreme pain and anguish and was hospitalized. She now faces additional upcoming surgeries to possibly remove her eye.
The lawsuit further claims that Bausch and Lomb committed acts of negligence through failing to remove the product from the market; continued to market and sell ReNu with Multiplus; failed to warn of all possible side effects and misrepresented and concealed the possible risks or dangers of use. As well, the manufacturer is also accused of strict liability through design, manufacture, and marketing defects and breach of express and implied warranties.
 Another suit claims that the FDA notified the healthcare industry about a recall of the product in November of 2006 but did not remove the product from stores. However, in the wake of countless contact lens solution users suffering potentially blinding eye infections, the agency is finally conducting a safety review and will convene a panel of experts this June, 2008 to "discuss general issues" with various lens cleaners and determine whether new testing or package labeling should be required.
Another suit claims that the FDA notified the healthcare industry about a recall of the product in November of 2006 but did not remove the product from stores. However, in the wake of countless contact lens solution users suffering potentially blinding eye infections, the agency is finally conducting a safety review and will convene a panel of experts this June, 2008 to "discuss general issues" with various lens cleaners and determine whether new testing or package labeling should be required.
Bausch & Lomb, based in Rochester, NY, was acquired by private-equity firm Warburg Pincus LLC in October, 2007. Analysts estimate that Bausch & Lomb could face potential liabilities of $1 billion plus over Renu lawsuits.
Last updated on
En Español SOLUCIóN RENU
FREE RENU LAWSUIT EVALUATION
Send your ReNu claim to a lawyer who will review your claim at NO COST or obligation.
GET LEGAL HELP NOW
GET LEGAL HELP NOW
ReNu Contact Lens Solution Recall
 In a warning letter back in October, 2006 the FDA criticized Bausch & Lomb for neglecting to report nearly 36 eye infections linked to Renu that occurred before sales of the product were suspended. The letter also cited the company for numerous violations at its Greenville, South Carolina manufacturing plant.
In a warning letter back in October, 2006 the FDA criticized Bausch & Lomb for neglecting to report nearly 36 eye infections linked to Renu that occurred before sales of the product were suspended. The letter also cited the company for numerous violations at its Greenville, South Carolina manufacturing plant. (In March 2007, Bausch & Lomb issued a voluntary recall of another contact lens product, its Renu Multiplus Solution--also manufactured at the same plant--this time for having trace amounts of iron, which could apparently lead to a shortened shelf life for the solution.)
ReNu Contact Lens Solution Lawsuits
 Bausch & Lomb now faces at least 400 consumer product liability lawsuits in the US from victims claiming injury from Renu, and it also faces lawsuits from shareholders. One suit alleges that the product was "unreasonably dangerous and defective" due to contamination and that Bausch & Lomb failed to warn consumers of the dangers associated with the product. The plaintiff suffered extreme pain and anguish and was hospitalized. She now faces additional upcoming surgeries to possibly remove her eye.
Bausch & Lomb now faces at least 400 consumer product liability lawsuits in the US from victims claiming injury from Renu, and it also faces lawsuits from shareholders. One suit alleges that the product was "unreasonably dangerous and defective" due to contamination and that Bausch & Lomb failed to warn consumers of the dangers associated with the product. The plaintiff suffered extreme pain and anguish and was hospitalized. She now faces additional upcoming surgeries to possibly remove her eye.The lawsuit further claims that Bausch and Lomb committed acts of negligence through failing to remove the product from the market; continued to market and sell ReNu with Multiplus; failed to warn of all possible side effects and misrepresented and concealed the possible risks or dangers of use. As well, the manufacturer is also accused of strict liability through design, manufacture, and marketing defects and breach of express and implied warranties.
 Another suit claims that the FDA notified the healthcare industry about a recall of the product in November of 2006 but did not remove the product from stores. However, in the wake of countless contact lens solution users suffering potentially blinding eye infections, the agency is finally conducting a safety review and will convene a panel of experts this June, 2008 to "discuss general issues" with various lens cleaners and determine whether new testing or package labeling should be required.
Another suit claims that the FDA notified the healthcare industry about a recall of the product in November of 2006 but did not remove the product from stores. However, in the wake of countless contact lens solution users suffering potentially blinding eye infections, the agency is finally conducting a safety review and will convene a panel of experts this June, 2008 to "discuss general issues" with various lens cleaners and determine whether new testing or package labeling should be required.Bausch & Lomb, based in Rochester, NY, was acquired by private-equity firm Warburg Pincus LLC in October, 2007. Analysts estimate that Bausch & Lomb could face potential liabilities of $1 billion plus over Renu lawsuits.
ReNu with MoistureLoc eye infection Legal Help
If you or a family member has suffered eye infections or eyesight damage after using ReNu with MoistureLoc contact lens solution, you may qualify for damages or remedies that might be awarded in a possible ReNu with MoistureLoc class action or lawsuit. Please fill out the form at right to submit your complaint.Last updated on
RENU LEGAL ARTICLES AND INTERVIEWS
ReNu Contact Lens Solution: Consumers Put at Risk

New Labeling Considered for ReNu with MoistureLoc

ReNu Lens Solution: Two Years After Recall, Lawsuits Keep Coming


June 19, 2008
Scientists and eye experts are urging the Food and Drug Administration (FDA) to consider stricter labeling guidelines for ReNu with MoistureLoc and other similar products. However, even though many of the recommendations may be adopted, the moves come too late for patients who were harmed by using the contact lens solution. Many are now investigating lawsuits alleging that ReNu with MoistureLoc lens solution was responsible for their injuries. READ MORE
New Labeling Considered for ReNu with MoistureLoc

June 11, 2008
The US Food and Drug Administration (FDA) has met with an advisory panel to discuss stricter standards for contact lens solutions such as ReNu with MoistureLoc. The panel, which involves a number of outside advisers to the FDA who are experts in ophthalmology, was convened after two massive contact lens solution recalls. Meanwhile, patients are still filing lawsuits against Bausch & Lomb, alleging that the company's lens solution caused severe damage to their eyes. READ MORE
ReNu Lens Solution: Two Years After Recall, Lawsuits Keep Coming

May 28, 2008
Imagine if someone poured sand into the front of your eye, then massaged your eye with a thousand tiny needles, then squeezed the eyeball. That's how one person described the pain associated with Fusarium Keratitis after using ReNu with MoistureLoc contact lens solution from Bausch & Lomb. READ MORE
READ MORE Drugs/Medical Settlements and Legal News
READ MORE Personal Injury Settlements and Legal News
READ MORE Personal Injury Settlements and Legal News

READER COMMENTS
Pennsylvania
on