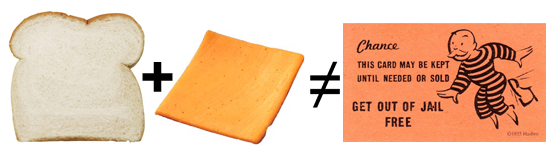Granny, in Bit of a Pickle, Offers Officer Grilled Cheese
October 4th, 2010. By LucyC
Welcome to Totally Tortelicious—a review of some of the more bizarre legal stories making news—and there’s certainly no shortage of them. 
Occifer Not Biting Granny’s Grilled Cheese. Most police officers who have pulled people over on suspicion of drunken driving have likely heard all kinds of excuses and been made all sorts of offers by the drivers in an attempt to get out of any potential charges. But this Grandmother (God help the grandchildren) has, I think come up with something completely original.
Sixty-five year old Elsie Wright O’Connor tried to bribe her way out of a DUI charge by offering to make the arresting officer a grilled cheese sandwich.
I wonder if that included a dill pickle…
Dear old Elsie, needless to say, failed in her attempt to persuade the officer that depriving society of a grandmother and the maker of a rather good grilled cheese are reasons enough to drop the charges. Hey, maybe he’d already eaten. Maybe he’s lactose intolerant…maybe she should have offered him a choice—egg salad and tuna fish are usually pretty popular.
But from the sound of it, the evidence was rather damning. Another driver, obviously ignorant of Elsie’s status and talents, reported her vehicle to the police as she nonchalantly swerved her way down the highway. When she was pulled over, Marion County Deputy Calvin Batts reportedly smelled a wee something on her breath—which he quickly surmised was in some way connected to the one and a half empty Skyy Vodka bottles rolling around in her Cadillac SUV. Enter the offer of a grilled cheese sandwich.
“Come on now, I’m a grandma, can’t you do something for me since I’m not that bad,” Batts Read the rest of this entry »
Recall Notice: Dirty Words for Medical Devices
October 1st, 2010. By Hunter West
 There are people walking around with faulty hip implants. If they can even walk at all. This month DePuy Orthopaedics, a division of the giant Johnson & Johnson family of companies, finally recalled troublesome hip implants after removing them from the market last year. DePuy undertook the latter response amidst a hail of criticism that the metal-on-metal implant featured a flawed design.
There are people walking around with faulty hip implants. If they can even walk at all. This month DePuy Orthopaedics, a division of the giant Johnson & Johnson family of companies, finally recalled troublesome hip implants after removing them from the market last year. DePuy undertook the latter response amidst a hail of criticism that the metal-on-metal implant featured a flawed design.
Okay, so recalls are nothing new. Cars and appliances are recalled all the time. But hip implants, and heart defibrillators are not cars and appliances. They are devices implanted in a patient’s body and not easily resolved.
Who could forget the pacemaker lead from a few years ago that was recalled? The device, which made fans of surgeons who liked the ease and flexibility of the new, thinner leads that proved easier to thread to the heart, was initially hailed as a breakthrough.
And then they started to break. Fracture, really. Hairline fractures. But just enough to affect the performance of the device. Patients who required life-giving shocks to keep their hearts going weren’t getting them; while others were injured or killed when a properly performing heart was suddenly hit with a rogue electric shock because the fractured lead impeded the flow of information from the heart to the pacemaker. Believing the heart to be failing (it wasn’t), the device went into action to re-engage a heart that in reality had not failed—often with tragic consequences.
Which begs the question—why does this keep happening?
Johnson & Johnson has been under siege of late, with a spate of recalls from the DePuy hip, to artificial knee joints, to medication such as Motrin. The company has had to shut down one of its manufacturing facilities due to a departure from GMP (good manufacturing practices), and there have been questions raised about the behemoth company’s oversight. The management and conduct of J&J has been the subject of congressional investigation.
But a larger question remains, and it is one not tied to any one company—but any company Read the rest of this entry »
Allergan and Botox or FDA: Who Has the Last Word?
September 30th, 2010. By janem
 As you may know, Allergan Inc. recently pled guilty to off-label promotion of its product Botox and dished out $600 million to resolve its criminal and civil liability suits. Allergan apparently made it a top corporate priority to maximize sales of Botox for headaches, pain, spasticity and juvenile cerebral palsy from 2000 to 2005—the time the FDA hadn’t approved Botox for marketing. Oh yeah, and Botox wasn’t approved to treat wrinkles either. So what was it approved for?
As you may know, Allergan Inc. recently pled guilty to off-label promotion of its product Botox and dished out $600 million to resolve its criminal and civil liability suits. Allergan apparently made it a top corporate priority to maximize sales of Botox for headaches, pain, spasticity and juvenile cerebral palsy from 2000 to 2005—the time the FDA hadn’t approved Botox for marketing. Oh yeah, and Botox wasn’t approved to treat wrinkles either. So what was it approved for?
In 1989, Botox was FDA-approved to treat strabismus (crossed eyes) and blepharospasm (involuntary eyelid muscle contraction). In 2000 and 2004, it was approved to treat cervical dystonia (involuntary neck muscle contraction) and primary axillary hyperhidrosis (excessive underarm sweating). Then in 2010, approval was given to treat adult upper-limb spasticity. That’s it.
But greed got the better of Allergan and it concocted a plan in 2003 to tap into the headache and pain market: It came up with the “CD/HA Initiative” (CD standing for on-label cervical dystonia) as a “rescue strategy”, it claimed that cervical dystonia was “underdiagnosed” and that doctors could diagnose cervical dystonia based on headache and pain symptoms, even when the doctor “doesn’t see any cervical dystonia.” So the FDA steps in and justice is served.
The civil penalties will be divided between federal and state health plans that were billed for unapproved uses of Botox, and five whistle-blowers who filed lawsuits in Georgia under the False Claims Act.
EXCEPT that Allergan seems to get the last word in: As part of the agreement, the company was required to drop its lawsuit filed against the FDA in October challenging a government rule that prohibits companies from marketing “off-label” promotions, i.e., unapproved uses.
“This is a good outcome that protects the American people,” said the FDA Commissioner. “The off-label promotion of drugs threatens public health and the regulatory framework of the FDA.”
HUH? Why did the FDA make a deal to drop Allergan’s off-label promotions suit—is the agency that unsure of its legal position, or does Allergan have deeper legal pockets? Or?? Curious minds would sure like to know…
Asbestos News Roundup: 9.30.10
September 30th, 2010. By LucyC
A roundup of recent asbestos-related news and information that you should be aware of.
Asbestos Lawsuits
Elyria, OH: The family of a Richard W. Fanslau, who died from asbestos related lung disease and lung cancer, has filed an asbestos lawsuit against Goodrich, Norfolk Southern Railway Co., and numerous other defendants. The suit claims that Fanslau was exposed to asbestos while working for the Nickel Plate Railroad and BF Goodrich, which led to his death. The family is suing for at least $25,000 in damages. (morningjournal.com)
Asbestos Hot Spots
Berkeley, CA: Children in an elementary school in California may have been exposed to asbestos while taking music and cooking classes. California state safety officials have verified reports of asbestos at the Washington Elementary School in Berkeley, and inspectors with the California Division of Occupational Safety and Health (Cal/OSHA) ordered that the room remain closed until the school could make a full assessment of the level of contamination.
The asbestos was discovered by part-time teacher Darwin Greenwell, who has a background in construction and real estate. Greenwell and other staff at the school had noticed that the carpet runners in the classroom had been removed, exposing the floor tiles underneath. The floor tiles appeared to be in poor condition, and a fine white dust covered the carpets near where the runners had been. Greenwell told a local newspaper that, when the children would dance during music class, “they would raise the dust.”
Greenwell was suspicious of the tiles and dust because they appeared similar to other tiles in other properties and that those types of tile usually contained asbestos.
In response to the room closure, the school district contacted a local asbestos abatement contractor, RGA Environmental, to conduct the remediation project. The workers removed the tiles and the adhesive, known as “mastic”, as both tested positive for asbestos. The room remains closed due to the dust still evident on many surfaces. (Berkeley Daily Planet.com)
San Bruno, CA: City, county and state health officials have expressed concern that some of Read the rest of this entry »
Ben & Jerry’s Agrees to Lose “All Natural” Claim; On to FRS…
September 30th, 2010. By LucyC
 Those bastions of all that is good about ice cream—Ben & Jerry’s—had their wrists slapped recently over the inappropriate use of the phrase “All Natural”. And they, unlike some other companies I will mention, had the good sense to do something about the false claim.
Those bastions of all that is good about ice cream—Ben & Jerry’s—had their wrists slapped recently over the inappropriate use of the phrase “All Natural”. And they, unlike some other companies I will mention, had the good sense to do something about the false claim.
Apparently, there are about 48 of Ben & Jerry’s products that aren’t ‘All Natural’, according to the Center for Science in the Public Interest (CSPI), a nutrition and food safety watchdog group based in Washington, DC.
In case you’re wondering just what the heck constitutes ‘natural’, information on the CSPI website states that “The U.S. Department of Agriculture, which regulates meat and poultry, lets products be labeled “natural” if they do not include artificial colors or ingredients, or are not more than “minimally processed,” by which the agency means a process that doesn’t fundamentally alter the raw ingredient. But the FDA, which regulates all other foods, has no such definition. It told CSPI several years ago that defining the term was “not among our enforcement priorities.”
Of course the irony in this is that the sugar and cream in ice cream will likely cause more harm to your arteries—not to mention your teeth—than most of the “unnatural products” in Ben & Jerry’s ice creams and frozen yogurts, products including alkalized cocoa, corn syrup, and partially hydrogenated soybean oil.
Nevertheless, Ben and Jerry’s, not wishing to tarnish their best of the best image, have agreed to phase out the use of “All Natural” claims on their labels.
Oh, that all manufacturers were so obliging. Not so in the instance of FRS Healthy Energy Drink, a line of products sold by the FRS Company and distributed by PepsiCo. The product Read the rest of this entry »
Archive by Category
- Accidents (24)
- Airlines (9)
- Asbestos Mesothelioma (262)
- Automotive (25)
- Celebrity (14)
- Class Action (84)
- Complaints/Comments (15)
- Consumer Fraud (84)
- Contest (2)
- Court of Public Opinion (5)
- Crazy Sh*t Lawyers See (61)
- Criminal Law (4)
- Defective Products (111)
- DePuy ASR Hip Recall (2)
- Discrimination (22)
- Drugs/Medical (248)
- Elder Care Abuse (4)
- Emerging Issues (462)
- Employment (54)
- Environment (52)
- Financial (28)
- Food Illness (15)
- Human/Civil Rights (4)
- Insecurities (5)
- Insurance (16)
- Intellectual Property (16)
- Internet/E-commerce (19)
- lawsuits (161)
- Lawyers (20)
- Lawyers Giving Back (43)
- Lex Levity (10)
- Personal Injury (106)
- Pleading Ignorance (53)
- Real Estate (2)
- Recall (6)
- Scam (3)
- Securities (13)
- Settlement (81)
- Tort Reform (2)
- Totally Tortelicious (81)
- Veterans (11)
- Whistleblower (9)