New Botox Warnings: Should Nicole Kidman be worried?
August 4th, 2009. By AbiK
 Hands down, Nicole Kidman has become bad Botox’s poster child. While she will never surpass the cherished position that Jocelyn Wildenstein (if you don’t know her, google her) holds in the annals of cosmetic plastic surgery, Nicole is definitely looking smooth while sporting an otherwise would-be scrunched brow look. But should she, and other Botox devotees, take notice of yesterday’s FDA update on Botulinum Toxin (aka Botox et al) safety?
Hands down, Nicole Kidman has become bad Botox’s poster child. While she will never surpass the cherished position that Jocelyn Wildenstein (if you don’t know her, google her) holds in the annals of cosmetic plastic surgery, Nicole is definitely looking smooth while sporting an otherwise would-be scrunched brow look. But should she, and other Botox devotees, take notice of yesterday’s FDA update on Botulinum Toxin (aka Botox et al) safety?
Well, before all of Hollywood heads for the cover of dimly lit rooms, the FDA update warns that Botulinum Toxin injections can spread from the area of injection to cause symptoms simillar to botulism; these include potentially life-threatening swallowing and breathing difficulties and even death.
The FDA points out that “symptoms have mostly been reported in children with cerebral palsy being treated with botulinum toxin for muscle spasticity, a use of the drugs that has not been approved by FDA.” Lucky for Nicole et al that Botox has been approved by the FDA for dermatological use. And, to date, no adverse event reports of distant Botox spread have been associated with its use for frown lines or excessive underarm sweating (yes, another approved use). Read the rest of this entry »
Avandia and Liver Damage: Who’s Keeping Score?
August 4th, 2009. By janem
 Researchers working with Public Citizen must feel like they’re banging their heads against the FDA wall when it comes to Avandia and liver failure. Their recent study of adverse events (called “Case series of liver failure associated with rosiglitazone and pioglitazone” ) reported 11 deaths due to liver toxicity between 1997 and 2006 associated with the use of Avandia. Public Citizen has also been petitioning the FDA since October, 2008, to ban Avandia, saying the drug’s risks outweigh its benefits.
Researchers working with Public Citizen must feel like they’re banging their heads against the FDA wall when it comes to Avandia and liver failure. Their recent study of adverse events (called “Case series of liver failure associated with rosiglitazone and pioglitazone” ) reported 11 deaths due to liver toxicity between 1997 and 2006 associated with the use of Avandia. Public Citizen has also been petitioning the FDA since October, 2008, to ban Avandia, saying the drug’s risks outweigh its benefits.
You’re probably thinking that 11 deaths are rather insignificant in the big picture: Public Citizen reported that in 2006, the number of prescriptions filled for the drug peaked at 13.2 million and dropped to 3.1 million in 2008, which means about 8,500 prescriptions a day are still being filled for Avandia.
But those same researchers have pointed out that reporting rates to the FDA are low; most patients who develop liver disease from Avandia likely never report it: They believe 1 in 44,000 patients who take Avandia are at risk for developing liver failure. And Dr. Sidney Wolfe, acting president of Public Citizen, director of Public Citizen’s Health Research Group and co-author of the report, said “The research [on Avandia and liver failure] is yet another indication that Avandia is too dangerous to remain on the market”.
Still, the FDA hasn’t taken this dangerous drug off the market, even though there are safer alternatives. Perhaps the agency is taking into account the manufacturer’s take on all this. According to latimesblogs.com (July 25, 2009), GlaxoSmithKline argues that no scientific study has linked the drug to liver toxicity. After all, 8500 prescriptions a day=cha ching…
Louisiana look out – Virginia’s on your Chinese Drywall tail
August 4th, 2009. By AbiK
And a few other states.
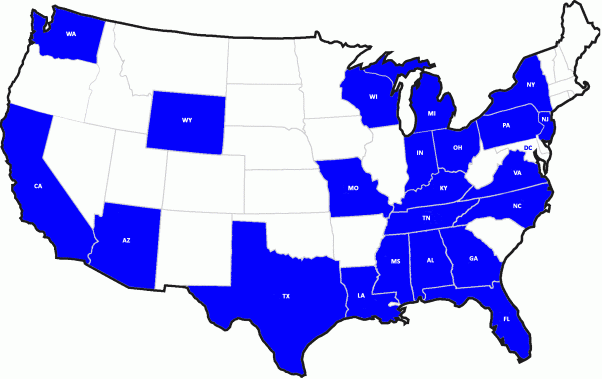
Seems Chinese drywall “creep” has expanded to a few more states since my last update (7/10/09) on this front. Now, the picture—which has that “separated at birth” look with a CNN election day map—shows a bit more blue. New states reporting incidents of Chinese drywall, according to the CPSC, include Indiana, Michigan and Pennsylvania.
And yes, Virginia, you’re hot on Louisiana’s tail on this front—not that you want to be. Virginia now has 23 reports in to trail Louisiana’s 96. Florida is still in the lead with 510 cases reported.
Heparin Lawsuits: Still Time to File
August 4th, 2009. By LucyC
 Between November 2007 and February 2008 reports of adverse health effects linked to heparin started to flood the media. By February 2008, the US Food and Drug Administration (FDA) and the Centers for Disease Control (CDC) had managed to identify the initial source of the problem, and consequently announced a recall of certain heparin products produced by Baxter Healthcare.
Between November 2007 and February 2008 reports of adverse health effects linked to heparin started to flood the media. By February 2008, the US Food and Drug Administration (FDA) and the Centers for Disease Control (CDC) had managed to identify the initial source of the problem, and consequently announced a recall of certain heparin products produced by Baxter Healthcare.
Heparin is used in a variety of medical applications, including cardiac procedures, dialysis and flush catheters. Because possible routes of exposure include injections, pre-filled syringes or IV bags, the potential for hundreds if not thousands of people to have been exposed to the potentially lethal product was enormous. And the numbers give some indication of that. Read the rest of this entry »
Corazon Aquino, Colonoscopy Prep & my $5,275 Colonoscopy
August 3rd, 2009. By AbiK
 This weekend’s passing of Corazon Aquino after her battle with colon cancer puts the disease back in the spotlight—particularly at a time when online reports estimate that anywhere from 50%- 60% of Americans over 50 years of age have not had a colonoscopy.
This weekend’s passing of Corazon Aquino after her battle with colon cancer puts the disease back in the spotlight—particularly at a time when online reports estimate that anywhere from 50%- 60% of Americans over 50 years of age have not had a colonoscopy.
At first, that statistic seems alarming. But here’s the thing—who wants a colonoscopy? I’ve already posted about how the colonoscopy prep options are like being between a rock and a hard place: either you gag down a gallon of wannabe fruit-flavored solution, or you potentially increase your risk of kidney failure by using the more palatable Osmo-Prep, Visicol or maybe Fleet EZ Prep.
Perhaps the biggest thing of all to swallow is the cost of a colonoscopy. Here’s the breakdown of my recent colonoscopic adventure:
Doctor Consultation: $375
Doctor Fee for Procedure: $2100
Facility Fee: $2800
Total: a whopping $5,275
And keep in mind that if you haven’t met your deductible, or if you’re paying out-of-network, you’ll be forking over a large chunk of that $5,275. Read the rest of this entry »
Archive by Category
- Accidents (24)
- Airlines (9)
- Asbestos Mesothelioma (262)
- Automotive (25)
- Celebrity (14)
- Class Action (84)
- Complaints/Comments (15)
- Consumer Fraud (84)
- Contest (2)
- Court of Public Opinion (5)
- Crazy Sh*t Lawyers See (61)
- Criminal Law (4)
- Defective Products (111)
- DePuy ASR Hip Recall (2)
- Discrimination (22)
- Drugs/Medical (248)
- Elder Care Abuse (4)
- Emerging Issues (462)
- Employment (54)
- Environment (52)
- Financial (28)
- Food Illness (15)
- Human/Civil Rights (4)
- Insecurities (5)
- Insurance (16)
- Intellectual Property (16)
- Internet/E-commerce (19)
- lawsuits (161)
- Lawyers (20)
- Lawyers Giving Back (43)
- Lex Levity (10)
- Personal Injury (106)
- Pleading Ignorance (53)
- Real Estate (2)
- Recall (6)
- Scam (3)
- Securities (13)
- Settlement (81)
- Tort Reform (2)
- Totally Tortelicious (81)
- Veterans (11)
- Whistleblower (9)