LAWSUITS NEWS & LEGAL INFORMATION
Celexa Heart Defects in Newborns
Celexa is a brand name for citalopram, a Selective Serotonin Reuptake Inhibitor (SSRI). Celexa is used to treat patients with major depressive disorder and obsessive compulsive disorder. However, some studies have found a link between Celexa and birth defects when pregnant women have used the antidepressant. These Celexa-related birth defects can be life-threatening. Lawsuits have been filed alleging patients were not properly warned about the risk of Celexa side effects.
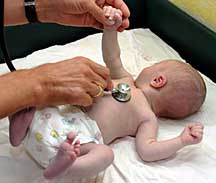 Recent studies indicate that Celexa can increase the risk of coronary birth defects when taken by pregnant women. One study, by researchers in the US and Denmark, compared 1,054 women who took SSRIs in the first three months of pregnancy with 150,000 others not on the drug. The study determined that women using the Selective Serotonin Re-uptake Inhibitors (SSRIs) in the first 12 weeks of gestation—when many may not know they are pregnant—have a 40 percent greater risk of their baby suffering malformations. Heart defects are 60 per cent more likely.
Recent studies indicate that Celexa can increase the risk of coronary birth defects when taken by pregnant women. One study, by researchers in the US and Denmark, compared 1,054 women who took SSRIs in the first three months of pregnancy with 150,000 others not on the drug. The study determined that women using the Selective Serotonin Re-uptake Inhibitors (SSRIs) in the first 12 weeks of gestation—when many may not know they are pregnant—have a 40 percent greater risk of their baby suffering malformations. Heart defects are 60 per cent more likely.
Celexa was developed by the Danish pharmaceutical firm, H. Lundbeck A/S and was introduced into the US market by Forest Laboratories and Parke-Davis in September 1998. It works by raising or restoring the patient's serotonin levels to relieve depression.
Currently in Kentucky, the first Celexa birth defects lawsuit alleges that Forest Laboratories has engaged in "repeated and persistent fraud" by misrepresenting, concealing and otherwise failing to disclose information concerning the safety and effectiveness of Celexa in treating pregnant women.
According to the lawsuit, the plaintiff in the case was prescribed Celexa during her first trimester of pregnancy and as a result, her baby was born with serious heart birth defects. Her son, born in May, 2006, was diagnosed with Shone's Complex, a form of congenital heart disease that consists of multiple anatomic defects that lead to the obstruction of blood flow from the left side of the heart to the body.
The lawsuit alleges that the drug maker "failed to warn the public and the medical community about the special risks of developing birth defects in newborns, cardiovascular defects, heart related birth defects, or other serious problems associated with the use of Celexa."
In May 2005, researchers from the University of Pittsburgh estimated in the Journal of American Medical Association, that in any given year at least 80,000 pregnant women in US are prescribed SSRIs, including Celexa.
Last updated on
En Español CELEXA DEFECTOS DE NACIMIENTO
FREE CELEXA LAWSUIT EVALUATION
Send your Celexa claim to a lawyer who will review your claim at NO COST or obligation.
GET LEGAL HELP NOW
GET LEGAL HELP NOW
Celexa Studies
 Recent studies indicate that Celexa can increase the risk of coronary birth defects when taken by pregnant women. One study, by researchers in the US and Denmark, compared 1,054 women who took SSRIs in the first three months of pregnancy with 150,000 others not on the drug. The study determined that women using the Selective Serotonin Re-uptake Inhibitors (SSRIs) in the first 12 weeks of gestation—when many may not know they are pregnant—have a 40 percent greater risk of their baby suffering malformations. Heart defects are 60 per cent more likely.
Recent studies indicate that Celexa can increase the risk of coronary birth defects when taken by pregnant women. One study, by researchers in the US and Denmark, compared 1,054 women who took SSRIs in the first three months of pregnancy with 150,000 others not on the drug. The study determined that women using the Selective Serotonin Re-uptake Inhibitors (SSRIs) in the first 12 weeks of gestation—when many may not know they are pregnant—have a 40 percent greater risk of their baby suffering malformations. Heart defects are 60 per cent more likely.
Celexa was developed by the Danish pharmaceutical firm, H. Lundbeck A/S and was introduced into the US market by Forest Laboratories and Parke-Davis in September 1998. It works by raising or restoring the patient's serotonin levels to relieve depression.
Celexa Lawsuits
According to the lawsuit, the plaintiff in the case was prescribed Celexa during her first trimester of pregnancy and as a result, her baby was born with serious heart birth defects. Her son, born in May, 2006, was diagnosed with Shone's Complex, a form of congenital heart disease that consists of multiple anatomic defects that lead to the obstruction of blood flow from the left side of the heart to the body.
The lawsuit alleges that the drug maker "failed to warn the public and the medical community about the special risks of developing birth defects in newborns, cardiovascular defects, heart related birth defects, or other serious problems associated with the use of Celexa."
In May 2005, researchers from the University of Pittsburgh estimated in the Journal of American Medical Association, that in any given year at least 80,000 pregnant women in US are prescribed SSRIs, including Celexa.
Celexa Heart Birth Defect Legal Help
If you took Celexa while pregnant, and your baby was born with heart defects, please click below to send your case to a lawyer who will review it at no cost.Last updated on
CELEXA LEGAL ARTICLES AND INTERVIEWS
Celexa Lawsuit Results in Settlement with Idaho
.jpg)
Study Suggests Link Between Antidepressant Use and Premature Birth

Studies Suggest Celexa Birth Defects Link, but Results Conflict

.jpg)
August 26, 2012
The widely-held position by the US Food and Drug Administration (FDA) that drugs are considered acceptable for use if the benefits for the intended patient outweigh the risks, has never been debated more stringently than with SSRI antidepressants and their relationship to pregnant women. Specifically Celexa (Citalopram), and the potential for Celexa birth defects. READ MORE
Study Suggests Link Between Antidepressant Use and Premature Birth

July 11, 2012
A study published in the American Journal of Obstetrics & Gynecology (5/2/12) suggests a link between use of antidepressants such as Celexa while pregnant and an increased risk of premature birth. Although the study does not examine the risk of Celexa birth defects or defects linked to other antidepressants, it does raise the issue of Celexa side effects in infants, and the effects of other antidepressants on newborns exposed to the drugs prior to birth. READ MORE
Studies Suggest Celexa Birth Defects Link, but Results Conflict

October 3, 2011
When it comes to pregnancy, there is a great deal of debate over whether women should be concerned about things such as Celexa birth defects. The debate comes in part because unlike with alcohol or smoking during pregnancy—which have been consistently shown to cause harm to a fetus—studies on Celexa side effects and other medication side effects in infants have been inconsistent. Although some studies have shown that SSRIs such as Celexa are linked to birth defects, other studies have not found the same results, leaving women wondering what medications, if any, they can take while pregnant. READ MORE
READ MORE Birth Injury and Birth Defect Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News
