LAWSUITS NEWS & LEGAL INFORMATION
Drug Eluting Stents
Were you looking for FDA Issues Warning for Transvascular Autonomic Modulation (TVAM) lawsuits?
By LAS Staff Writer
Lawyers are currently investigating the numbers of stent-related cardiac events linked to drug eluting stents. Drug eluting stents are also known as medicated or drug-coated stents. Medicated stents are, unlike bare metal stents, coated in drugs intended to inhibit cell growth to prevent restenosis (re-blocking of the artery).
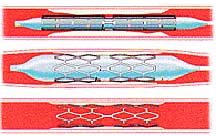 Manufacturers of these stents include Boston Scientific (Taxus stent) and Cordis, a subsidiary of Johnson & Johnson (Cypher stent). Medicated stents are increasingly common, projected to represent 88% of all stents sold in the US in 2005.
Manufacturers of these stents include Boston Scientific (Taxus stent) and Cordis, a subsidiary of Johnson & Johnson (Cypher stent). Medicated stents are increasingly common, projected to represent 88% of all stents sold in the US in 2005.
Drug Eluting Stents have been linked to side effects such as thrombosis and severe allergic reactions.
Stents were originally developed to open arteries clogged with plaque. A treatment called angioplasty was developed whereby doctors expanded clogged arteries with tiny balloons, but about half the time the arteries re-closed.
In an attempt to solve the problem, doctors used the balloon to expand a tiny wire mesh sleeve, called a stent, inside the artery to keep it open. This helped a bit, but in 20-30% of cases, cells grew over the wire and the arteries re-closed.
The next solution was to coat the stents with a drug that prevented cell growth. These drug eluting stents (DES) cut the restenosis rate to about 5%. However, a new problem developed - by stopping cell growth, pieces of the stent could stick out of the artery and create a perfect place for a blood clot to form and cause a fatal heart attack.
The drug-coated stents have also been linked to a higher death rate due to cardiac events such as heart attack when compared to metal stents without a drug coating. The associated blood clots may occur up to thirty days after the heart stent was implanted. It is estimated that 2,000 Americans die each year due to drug eluting stents.
While allergic reactions may also occur, including pain, hives, and fever, along with difficult breathing and blood pressure issues, the law firm is interested in investigating only those cases that relate to post-stent implantation cardiac events. The strongest cases involve death with autopsy showing a blood clot inside the stent.
Last updated on
FREE DRUG ELUTING STENTS LAWSUIT EVALUATION
Send your Drug Eluting Stents claim to a lawyer who will review your claim at NO COST or obligation.
GET LEGAL HELP NOW
GET LEGAL HELP NOW
Drug Eluting Stents' Side Effects
 Manufacturers of these stents include Boston Scientific (Taxus stent) and Cordis, a subsidiary of Johnson & Johnson (Cypher stent). Medicated stents are increasingly common, projected to represent 88% of all stents sold in the US in 2005.
Manufacturers of these stents include Boston Scientific (Taxus stent) and Cordis, a subsidiary of Johnson & Johnson (Cypher stent). Medicated stents are increasingly common, projected to represent 88% of all stents sold in the US in 2005. Drug Eluting Stents have been linked to side effects such as thrombosis and severe allergic reactions.
Stents were originally developed to open arteries clogged with plaque. A treatment called angioplasty was developed whereby doctors expanded clogged arteries with tiny balloons, but about half the time the arteries re-closed.
In an attempt to solve the problem, doctors used the balloon to expand a tiny wire mesh sleeve, called a stent, inside the artery to keep it open. This helped a bit, but in 20-30% of cases, cells grew over the wire and the arteries re-closed.
The next solution was to coat the stents with a drug that prevented cell growth. These drug eluting stents (DES) cut the restenosis rate to about 5%. However, a new problem developed - by stopping cell growth, pieces of the stent could stick out of the artery and create a perfect place for a blood clot to form and cause a fatal heart attack.
The drug-coated stents have also been linked to a higher death rate due to cardiac events such as heart attack when compared to metal stents without a drug coating. The associated blood clots may occur up to thirty days after the heart stent was implanted. It is estimated that 2,000 Americans die each year due to drug eluting stents.
While allergic reactions may also occur, including pain, hives, and fever, along with difficult breathing and blood pressure issues, the law firm is interested in investigating only those cases that relate to post-stent implantation cardiac events. The strongest cases involve death with autopsy showing a blood clot inside the stent.
Drug Eluting Stent Lawyers
If you or a loved one has suffered a heart attack or stroke after having a drug eluting stent implanted, please click the link below to send your Drug Eluting Stents claim to a lawyer for a free evaluation.Last updated on
DRUG ELUTING STENTS LEGAL ARTICLES AND INTERVIEWS
Angioplasty TVAM, Doctors and the FDA

Washington, DC: Angioplasty was designed as a safe alternative to open heart surgery, but a procedure using balloon angioplasty devices called transvascular autonomic modulation (TVAM) is not safe, which the FDA has warned of repeatedly. Some doctors, however, believe its benefits outweigh the risks involved.
The agency in March 2017 issued a warning to patients with autonomic dysfunction (nervous system disorders), including Parkinson’s disease, multiple sclerosis (MS), fibromyalgia, multiple system atrophy and more, and doctors who use balloon angioplasty devices to treat them. TVAM has not been approved or formally studied and it could put patients at risk of serious complications. But one doctor continues to use the device “off-label”, saying the benefits of this procedure are “usually seen immediately.” He also treats Lyme disease with TVAM rather than antibiotics and explains it in a video.
In September 2016 the FDA put Dr. Arata of Newport Beach, California on notice by way of “Initiation of Disqualification Proceedings and Opportunity to Explain”
(NIDPOE), to determine whether he should be disqualified from receiving investigational products under FDA’s regulations. Arata claims the angioplasty TVAM procedure treats the signs and symptoms of autonomic dysfunction in some neurological disorders, but the FDA says it has no data to indicate such claims. Rather, the agency says that TVAM is an experimental treatment that has been associated with at least one death.
Back in May 2012, theFDA warned of the risks involved with a similar procedure. The same balloon angioplasty devices were used to treat chronic cerebrospinal venous insufficiency (CCSVI). Reports of serious complications included one patient who died from bleeding in the brain and another patient suffered permanent paralysis from a stroke after CCSVI treatment. Are doctors aware of the FDA reports regarding balloon angioplasty and choose to use the device off-label, potentially putting their patients at risk? The agency received a report after this 2012 warning that a balloon ruptured while being placed in a patient’s jugular vein. The device moved to the patient's lung, where it was surgically removed.
A warning letter (February 2012) was sent to Dr. Manish Mehta, whose practice in Albany, New York was investigated by the FDA. “The inspection was conducted under a program designed to ensure that data and information contained in requests for Investigational Device Exemptions (IDE), Premarket Approval applications, and Premarket Notification (510(k)) submissions are scientifically valid and accurate. Another objective of the program is to ensure that human subjects are protected from undue hazard or risk during the course of scientific investigations.” The agency found that Mehta had violated a clinical study by failing to submit correct documentation before patients were treated with the balloon angioplasty device.
Back to March 2017: Canadian researchers at the University of British Columbia and Vancouver Coastal Health reported results from a study that involved 104 patients with MS treated with angioplasty TVAM, according to the Associated Press. The patients did no better with TVAM than those without the procedure. And the FDA’s case against Arata is on hold: to be continued…
READ MORE
FDA Warns Against Off Label Angioplasty Treatment Known as TVAM

Jury Still Out on Safety of Drug Eluting Stents


May 19, 2017
Washington, DC: Angioplasty was designed as a safe alternative to open heart surgery, but a procedure using balloon angioplasty devices called transvascular autonomic modulation (TVAM) is not safe, which the FDA has warned of repeatedly. Some doctors, however, believe its benefits outweigh the risks involved.
The agency in March 2017 issued a warning to patients with autonomic dysfunction (nervous system disorders), including Parkinson’s disease, multiple sclerosis (MS), fibromyalgia, multiple system atrophy and more, and doctors who use balloon angioplasty devices to treat them. TVAM has not been approved or formally studied and it could put patients at risk of serious complications. But one doctor continues to use the device “off-label”, saying the benefits of this procedure are “usually seen immediately.” He also treats Lyme disease with TVAM rather than antibiotics and explains it in a video.
In September 2016 the FDA put Dr. Arata of Newport Beach, California on notice by way of “Initiation of Disqualification Proceedings and Opportunity to Explain”
(NIDPOE), to determine whether he should be disqualified from receiving investigational products under FDA’s regulations. Arata claims the angioplasty TVAM procedure treats the signs and symptoms of autonomic dysfunction in some neurological disorders, but the FDA says it has no data to indicate such claims. Rather, the agency says that TVAM is an experimental treatment that has been associated with at least one death.
Back in May 2012, the
A warning letter (February 2012) was sent to Dr. Manish Mehta, whose practice in Albany, New York was investigated by the FDA. “The inspection was conducted under a program designed to ensure that data and information contained in requests for Investigational Device Exemptions (IDE), Premarket Approval applications, and Premarket Notification (510(k)) submissions are scientifically valid and accurate. Another objective of the program is to ensure that human subjects are protected from undue hazard or risk during the course of scientific investigations.” The agency found that Mehta had violated a clinical study by failing to submit correct documentation before patients were treated with the balloon angioplasty device.
Back to March 2017: Canadian researchers at the University of British Columbia and Vancouver Coastal Health reported results from a study that involved 104 patients with MS treated with angioplasty TVAM, according to the Associated Press. The patients did no better with TVAM than those without the procedure. And the FDA’s case against Arata is on hold: to be continued…
READ MORE
FDA Warns Against Off Label Angioplasty Treatment Known as TVAM

April 17, 2017
Dallas, TX The FDA is taking the unusual step of calling out a specific doctor and warning people to steer clear of the use of TVAM angioplasty as a treatment for serious illnesses such as Parkinson’s disease, Multiple Sclerosis and other autonomic disorders. READ MORE
Jury Still Out on Safety of Drug Eluting Stents

October 20, 2008
When the US Food and Drug Administration (FDA) approved 'Endeavor,' the drug-coated Medtronic stent earlier this year, it did so amidst a storm of controversy as to whether or not the stents promote potentially dire blood clots in heart attack patients. Drug eluting stents are still under FDA scrutiny, but as 2008 dawned there was little conclusive evidence to prove the suspicion as fact. READ MORE
READ MORE Drugs/Medical Settlements and Legal News

READER COMMENTS
Brian Oliveira
on
Manitoba
on
The heart attack had damaged my heart and was attributed to a blood clot that formed in my Taxus stent. I am now on Warfarin to ensure no clots form in my heart chamber and then am looking at a lifetime of Plavix and aspirin. I have also lost my drivers license for the next 30 days. I also face a future of uncertainty regarding the Taxus stent and possible side effects and risks.