LAWSUITS NEWS & LEGAL INFORMATION
Fosamax Lawsuits
Read our Fosamax FAQ
Were you looking for Fosamax Stroke lawsuits?
Fosamax is prescribed to increase bone density in patients (mainly menopausal women) with osteoporosis. Fosamax side effects include Fosamax osteonecrosis, also known as Fosamax dead jaw, and recently Fosamax femur fractures. Osteonecrosis of the jaw (ONJ) is a disease in which bone tissue in the jaw does not heal after minor traumas such as tooth extraction or oral surgery.
 Since 2006, hundreds of Fosamax ONJ lawsuits against Merck, the manufacturer of Fosamax (the brand name for Alendronate), claim that rather than increasing bone density and strengthening bones, in some cases Fosamax has actually caused a deterioration of the jaw bone or contributed to femur fractures.
Since 2006, hundreds of Fosamax ONJ lawsuits against Merck, the manufacturer of Fosamax (the brand name for Alendronate), claim that rather than increasing bone density and strengthening bones, in some cases Fosamax has actually caused a deterioration of the jaw bone or contributed to femur fractures.
Fosamax belongs to a class of drugs called bisphosphonates--which also includes drugs Actonel, Boniva and Reclast among others--that are prescribed to help prevent the loss of bone mass and are used to treat osteoporosis and similar diseases.
Fosamax was approved by the FDA in 1995 to treat post-menopausal osteoporosis and Paget's disease of bone: a chronic, focal skeletal disorder characterized by greatly increased and disorderly bone remodeling. Since its approval, Fosamax has been taken by nearly 10 million men and women.
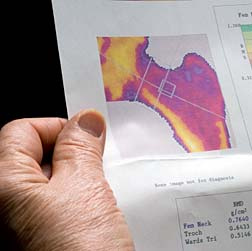
In 2008, researchers at Cornell University Medical School released a major study revealing that Fosamax patients are more than 125 times as likely to suffer non-traumatic femur fractures than patients who have not taken Fosamax.
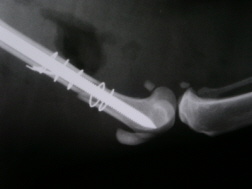 Other studies have suggested that patients using Fosamax may be at risk for increased risk of bone fracture, particularly spontaneous femur fractures. Reports published in the Journal of Clinical Endocrinology and Metabolism, the Journal of Orthopedic Trauma and other medical journals have warned that the drug produces an "increased susceptibility to, and delayed healing of, nonspinal fractures."
Other studies have suggested that patients using Fosamax may be at risk for increased risk of bone fracture, particularly spontaneous femur fractures. Reports published in the Journal of Clinical Endocrinology and Metabolism, the Journal of Orthopedic Trauma and other medical journals have warned that the drug produces an "increased susceptibility to, and delayed healing of, nonspinal fractures."
But it took the March 8, 2010, edition of ABC World News Tonight to fully expose the risk of low-energy, non-traumatic femur fractures for patients who take Fosamax for extended periods of time.
 Dr. Robert Bunning, a rheumatologist at National Rehabilitation Hospital in Washington DC, said that in all of the 50 to 60 reported cases, most patients had been taking Fosamax or another type of bisphosphonate for more than five years.
Dr. Robert Bunning, a rheumatologist at National Rehabilitation Hospital in Washington DC, said that in all of the 50 to 60 reported cases, most patients had been taking Fosamax or another type of bisphosphonate for more than five years.
Merck, the manufacturer of Fosamax, is reported to have said there has been no "causal link" demonstrated between long-term use of Fosamax and fractures. Clinical studies conducted by Merck have not shown any increased risk of fracture in any part of the body. On its website, however, Merck stated that clinical trials in postmenopausal women were of two or three years' duration. Other clinical studies were 3 and 4 years in duration, at the most.
Merck also states that "…after you start taking Fosamax, even though you won't see or feel a difference and that for fosamax to continue to work, you need to keep taking it."
A spokesperson with the Food and Drug Administration, FDA, said the agency "is aware of this issue and are actively investigating it."
More than 2,400 patients taking bone building medications, however, have reported cases of ONJ. A connection was discovered between the class of drugs known as bisphosphonates and a serious bone condition called Osteonecrosis of the Jaw (ONJ). This finding, published in the Journal of Oral and Maxillofacial Surgeons, showed the side effects of Fosamax may include ONJ. The USC School of Dentistry speculates that bisphosphonates—including Fosamax-- make it easier for bacteria to adhere to bone that is exposed after a tooth extraction or dental surgery, which leads to ONJ.
 The FDA and drug manufacturer Novartis issued warnings to health professionals in September 2004 about the potential of ONJ due to the use of bisphosphonates in chemotherapy patients. Most patients are treated with a more concentrated form of the drug, usually intravenously. While no statement was made about milder forms of the class, like Fosamax, many researchers see the potential for similar side effects.
The FDA and drug manufacturer Novartis issued warnings to health professionals in September 2004 about the potential of ONJ due to the use of bisphosphonates in chemotherapy patients. Most patients are treated with a more concentrated form of the drug, usually intravenously. While no statement was made about milder forms of the class, like Fosamax, many researchers see the potential for similar side effects.
Osteonecrosis of the jaw is a disease in which bone tissue in the jaw does not heal after minor traumas. Dental extractions that cause bone to become exposed can lead to fractures and infections often requiring long-term antibiotic therapy and surgery to remove the dead and dying bone tissue. Some researchers and pharmaceutical experts state that prevention and early treatment of patients using Fosamax is critical in the preservation of a healthy jaw bone. Individuals using Fosamax and other bisphosphonates should try to avoid tooth extractions and other major dental work while taking these medications.
Symptoms of Fosamax dead jaw include irregular sore with exposed bone, pain or swelling in the infected jaw; infection, possibly with pus; and/or an altered sensation, such as numbness or a heavy sensation. The highest risk factors for ONJ are:
 In July, 2011, the FDA announced it was reviewing data concerning a potential link between the use of bisphosphonates and the development of esophageal cancer. The agency noted that conflicting results had been seen in two studies of bisphosphonates and esophageal cancer, leading the FDA to conduct a review. One study found no link between the use of bisphosphonates and esophageal cancer, while the other study found approximately double the risk of esophageal cancer in patients who had more than ten bisphosphonate prescriptions or who had used bisphosphonates for more than three years. The FDA has reported a number of patients using Fosamax who developed esophageal tumors since its approval, including at least 8 deaths.
In July, 2011, the FDA announced it was reviewing data concerning a potential link between the use of bisphosphonates and the development of esophageal cancer. The agency noted that conflicting results had been seen in two studies of bisphosphonates and esophageal cancer, leading the FDA to conduct a review. One study found no link between the use of bisphosphonates and esophageal cancer, while the other study found approximately double the risk of esophageal cancer in patients who had more than ten bisphosphonate prescriptions or who had used bisphosphonates for more than three years. The FDA has reported a number of patients using Fosamax who developed esophageal tumors since its approval, including at least 8 deaths.
The New England Journal of Medicine (January 2009) said that the FDA received at least 23 reports of Fosamax esophageal cancer by May 2008, and another 21 reports of esophageal tumors among users of Fosamax were filed in Europe and Japan.
Esophageal cancer affects the tube between the throat and the stomach. As the tumors grow, symptoms may include difficulty swallowing, loss of weight and coughing blood.
In September 2011, two FDA advisory panels recommended strengthening the warnings on Fosamax but did not recommend what the new warnings should say nor did they recommend limiting use of the drugs to five years. In a 45-page report issued prior to the advisory panel meetings, FDA staff said studies suggest no significant benefit of continuing use of bisphosphonate drugs beyond five years. According to the FDA report, approximately nine percent of bisphosphonate users take the medications for longer than three years, with one percent using them longer than five years. The panels did recommend analyzing why the medications are prescribed for women who do not have osteoporosis. A revised label is expected later in 2011.
Merck's initial side effects stated that "side effects observed in clinical trials were generally mild. The most commonly reported drug-related side effects in subjects taking Fosamax were abdominal and musculoskeletal. Less frequently reported were other digestive disturbances such as nausea, heartburn, and irritation or pain of the esophagus"
 Merck later listed the following side effects in post-marketing use:
Merck later listed the following side effects in post-marketing use:
Gastrointestinal: esophagitis, esophageal erosions, esophageal ulcers, rarely esophageal stricture or perforation, and oropharyngeal ulceration. Gastric or duodenal ulcers, some severe and with complications, have also been reported.
Localized osteonecrosis of the jaw, generally associated with tooth extraction and/or local infectionwith delayed healing, has been reported rarely
Musculoskeletal: bone, joint, and/or muscle pain, occasionally severe, and rarely incapacitating; joint swelling; low-energy femoral shaft and subtrochanteric fractures.
In March 2010, Merck released a statement finally acknowledging the link between Fosamax and ONJ and provided information for ONJ in its "Adverse Reactions" section of the package circular as well as in the patient package insert.
It also adds: "FOSAMAX should not be used if the patient has certain disorders of the esophagus that delay emptying or if the patient is unable to stand or sit upright for at least 30 minutes."
 Merck included post-marketing experiences on ONJ and thigh fractures. Under its Adverse Reactions heading, the Fosamax Plus D label now includes post-marketing experience showing localized osteonecrosis of the jaw, generally associated with tooth extraction and/or local infection with delayed healing, has been reported in some patients, as has low-energy femoral shaft and subtrochanteric fractures.
Merck included post-marketing experiences on ONJ and thigh fractures. Under its Adverse Reactions heading, the Fosamax Plus D label now includes post-marketing experience showing localized osteonecrosis of the jaw, generally associated with tooth extraction and/or local infection with delayed healing, has been reported in some patients, as has low-energy femoral shaft and subtrochanteric fractures.
Prior to the new labelling, Merck had removed from the Fosamax Precautions the FDA's proposed language indicating that osteonecrosis of the jaw had occurred in patients taking oral bisphosphonates, such as Fosamax.
The updated labeling does not include studies by the Cornell University Medical School (above).
 In July 2005 Merck added a warning on page 13 of a 22-page document it distributed to pharmacies.
In July 2005 Merck added a warning on page 13 of a 22-page document it distributed to pharmacies.
The Merck website stated the following: [Fosamax] may cause jaw-bone problems in some people. Jaw-bone problems may include infection and delayed healing after teeth are pulled…improvement in bone density may be observed as early as 3 months after you start taking Fosamax, even though you won't see or feel a difference and that for fosamax to continue to work, you need to keep taking it.
The FDA ordered the makers of Fosamax and other bisphosphonates to list osteonecrosis in its precautions on package inserts. However, a lawsuit filed in a US District Court alleged that Merck deliberately concealed the drug's adverse side effects from doctors and patients and that Merck knew Fosamax could cause ONJ but still neglected to comply with the FDA's request to add a Fosamax warning to all drug labeling.
In April 2012, a study was released suggesting first-time users of Fosamax and other oral bisphosphonates may be at increased risk for serious inflammatory eye disease. The study, published in the Canadian Medical Association Journal (04/02/12), suggested that first-time users of the medications are at an increased risk of scleritis and uveitis. Although both conditions can be treated, if they remain untreated they can cause permanent eye damage.
 Hundreds of individual lawsuits—and at least two class action lawsuits--have been filed against Merck & Co. relating to Fosamax and its association with osteonecrosis of the jaw—a painful and often disfiguring disorder without a cure.
Hundreds of individual lawsuits—and at least two class action lawsuits--have been filed against Merck & Co. relating to Fosamax and its association with osteonecrosis of the jaw—a painful and often disfiguring disorder without a cure.
In April 2012, Merck announced it had won its fifth of six lawsuits concerning Fosamax. According to the drugmaker, a jury found that the plaintiff had medical conditions that increased the likelihood she would develop jaw problems after dental surgery, dismissing her claims that Fosamax caused her jaw problems.
2006: In April 2006, a class action suit was filed in U.S. District Court at Fort Meyers, Florida against Merck, alleging that Fosamax caused, in some cases, osteonecrosis of the jaw.
2007: A class action was filed against Merck Frosst Canada in 2007, claiming the company failed to adequately warn that one of its medications had been associated with a higher risk of developing decay of the jaw bone. The class action also named Merck & Co., the New Jersey parent company of Merck Frosst.
Merck said it had put aside $48 million to set up a defense fund for cases alleging Fosamax causes osteonecrosis, or dead jaw. The bulk of cases were filed in federal court. By May 2008 Merck faced about 100 new lawsuits claiming the company failed to warn that Fosamax may cause an irreversible jaw-rot.
2008: In July 2008 The United States District Court for the Southern District of New York remanded several cases to state courts in the States of California, Florida, and Illinois. The District Court also taxed costs and fees against Merck for the removal of the cases to federal court.
2010: The federal District courthouse in Manhattan scheduled the Boles v Merck retrial for June 7, 2010. Shirley Boles' lawyers intend to call a Merck witness to establish that by 2007, Merck had been clearly told by FDA to revise the Fosamax labeling, and to bring it into line with the widely-accepted (WHO and FDA) definitions of what constitutes osteoporosis.
In January 29, 2010 a Manhattan federal judge refused to dismiss a lawsuit alleging that Merck's Fosamax caused jaw damage to an Indiana woman during the nearly eight years she took the pill.
These cases all claim that Merck failed to adequately research Fosamax and warn about the risk of jaw problems. There are approximately 900 lawsuits currently pending against Merck & Co.
Despite Merck's claims of safety, more than 2,400 people have experienced jaw death from taking Fosamax, including cases where the jawbone rots and dies. Anyone who has taken Fosamax and experienced adverse side effects, especially relating to osteonecrosis of the jaw or femur fractures, may be eligible to become involved in litigation against Merck & Co.
According to data from IMS Heatlh, in 2008 bisphosphonate sales exceeded $3.5 billion.In the same year, over 37 million prescriptions were written for the osteoporosis medications. Merck reported that, since their market introduction, over 225 million total prescriptions have been dispensed for FOSAMAX/FOSAMAX Plus D, Actonel (risedronate) and Boniva (ibandronate) in the US alone.
Last updated on
FREE FOSAMAX LAWSUIT EVALUATION
Send your Fosamax claim to a lawyer who will review your claim at NO COST or obligation.
GET LEGAL HELP NOW
GET LEGAL HELP NOW
Fosamax
 Since 2006, hundreds of Fosamax ONJ lawsuits against Merck, the manufacturer of Fosamax (the brand name for Alendronate), claim that rather than increasing bone density and strengthening bones, in some cases Fosamax has actually caused a deterioration of the jaw bone or contributed to femur fractures.
Since 2006, hundreds of Fosamax ONJ lawsuits against Merck, the manufacturer of Fosamax (the brand name for Alendronate), claim that rather than increasing bone density and strengthening bones, in some cases Fosamax has actually caused a deterioration of the jaw bone or contributed to femur fractures.Fosamax belongs to a class of drugs called bisphosphonates--which also includes drugs Actonel, Boniva and Reclast among others--that are prescribed to help prevent the loss of bone mass and are used to treat osteoporosis and similar diseases.
Fosamax was approved by the FDA in 1995 to treat post-menopausal osteoporosis and Paget's disease of bone: a chronic, focal skeletal disorder characterized by greatly increased and disorderly bone remodeling. Since its approval, Fosamax has been taken by nearly 10 million men and women.
Fosamax Femur Fractures
 Other studies have suggested that patients using Fosamax may be at risk for increased risk of bone fracture, particularly spontaneous femur fractures. Reports published in the Journal of Clinical Endocrinology and Metabolism, the Journal of Orthopedic Trauma and other medical journals have warned that the drug produces an "increased susceptibility to, and delayed healing of, nonspinal fractures."
Other studies have suggested that patients using Fosamax may be at risk for increased risk of bone fracture, particularly spontaneous femur fractures. Reports published in the Journal of Clinical Endocrinology and Metabolism, the Journal of Orthopedic Trauma and other medical journals have warned that the drug produces an "increased susceptibility to, and delayed healing of, nonspinal fractures."But it took the March 8, 2010, edition of ABC World News Tonight to fully expose the risk of low-energy, non-traumatic femur fractures for patients who take Fosamax for extended periods of time.
 Dr. Robert Bunning, a rheumatologist at National Rehabilitation Hospital in Washington DC, said that in all of the 50 to 60 reported cases, most patients had been taking Fosamax or another type of bisphosphonate for more than five years.
Dr. Robert Bunning, a rheumatologist at National Rehabilitation Hospital in Washington DC, said that in all of the 50 to 60 reported cases, most patients had been taking Fosamax or another type of bisphosphonate for more than five years.Merck, the manufacturer of Fosamax, is reported to have said there has been no "causal link" demonstrated between long-term use of Fosamax and fractures. Clinical studies conducted by Merck have not shown any increased risk of fracture in any part of the body. On its website, however, Merck stated that clinical trials in postmenopausal women were of two or three years' duration. Other clinical studies were 3 and 4 years in duration, at the most.
Merck also states that "…after you start taking Fosamax, even though you won't see or feel a difference and that for fosamax to continue to work, you need to keep taking it."
A spokesperson with the Food and Drug Administration, FDA, said the agency "is aware of this issue and are actively investigating it."
Fosamax ONJ
 The FDA and drug manufacturer Novartis issued warnings to health professionals in September 2004 about the potential of ONJ due to the use of bisphosphonates in chemotherapy patients. Most patients are treated with a more concentrated form of the drug, usually intravenously. While no statement was made about milder forms of the class, like Fosamax, many researchers see the potential for similar side effects.
The FDA and drug manufacturer Novartis issued warnings to health professionals in September 2004 about the potential of ONJ due to the use of bisphosphonates in chemotherapy patients. Most patients are treated with a more concentrated form of the drug, usually intravenously. While no statement was made about milder forms of the class, like Fosamax, many researchers see the potential for similar side effects.Osteonecrosis of the jaw is a disease in which bone tissue in the jaw does not heal after minor traumas. Dental extractions that cause bone to become exposed can lead to fractures and infections often requiring long-term antibiotic therapy and surgery to remove the dead and dying bone tissue. Some researchers and pharmaceutical experts state that prevention and early treatment of patients using Fosamax is critical in the preservation of a healthy jaw bone. Individuals using Fosamax and other bisphosphonates should try to avoid tooth extractions and other major dental work while taking these medications.
Symptoms of Fosamax dead jaw include irregular sore with exposed bone, pain or swelling in the infected jaw; infection, possibly with pus; and/or an altered sensation, such as numbness or a heavy sensation. The highest risk factors for ONJ are:
- taking bisphosphonates, especially in IV form
- concurrent use of steroids
- previous history of cancer, osteoporosis or Paget's disease
- traumatic dental procedure, such as tooth extraction or dental implants.
Fosamax Esophageal Cancer
 In July, 2011, the FDA announced it was reviewing data concerning a potential link between the use of bisphosphonates and the development of esophageal cancer. The agency noted that conflicting results had been seen in two studies of bisphosphonates and esophageal cancer, leading the FDA to conduct a review. One study found no link between the use of bisphosphonates and esophageal cancer, while the other study found approximately double the risk of esophageal cancer in patients who had more than ten bisphosphonate prescriptions or who had used bisphosphonates for more than three years. The FDA has reported a number of patients using Fosamax who developed esophageal tumors since its approval, including at least 8 deaths.
In July, 2011, the FDA announced it was reviewing data concerning a potential link between the use of bisphosphonates and the development of esophageal cancer. The agency noted that conflicting results had been seen in two studies of bisphosphonates and esophageal cancer, leading the FDA to conduct a review. One study found no link between the use of bisphosphonates and esophageal cancer, while the other study found approximately double the risk of esophageal cancer in patients who had more than ten bisphosphonate prescriptions or who had used bisphosphonates for more than three years. The FDA has reported a number of patients using Fosamax who developed esophageal tumors since its approval, including at least 8 deaths.The New England Journal of Medicine (January 2009) said that the FDA received at least 23 reports of Fosamax esophageal cancer by May 2008, and another 21 reports of esophageal tumors among users of Fosamax were filed in Europe and Japan.
Esophageal cancer affects the tube between the throat and the stomach. As the tumors grow, symptoms may include difficulty swallowing, loss of weight and coughing blood.
Fosamax Side Effects Warnings
Fosamax Side Effects According to Merck
 Merck later listed the following side effects in post-marketing use:
Merck later listed the following side effects in post-marketing use:Gastrointestinal: esophagitis, esophageal erosions, esophageal ulcers, rarely esophageal stricture or perforation, and oropharyngeal ulceration. Gastric or duodenal ulcers, some severe and with complications, have also been reported.
Localized osteonecrosis of the jaw, generally associated with tooth extraction and/or local infectionwith delayed healing, has been reported rarely
Musculoskeletal: bone, joint, and/or muscle pain, occasionally severe, and rarely incapacitating; joint swelling; low-energy femoral shaft and subtrochanteric fractures.
Fosamax Re-labeling
It also adds: "FOSAMAX should not be used if the patient has certain disorders of the esophagus that delay emptying or if the patient is unable to stand or sit upright for at least 30 minutes."
 Merck included post-marketing experiences on ONJ and thigh fractures. Under its Adverse Reactions heading, the Fosamax Plus D label now includes post-marketing experience showing localized osteonecrosis of the jaw, generally associated with tooth extraction and/or local infection with delayed healing, has been reported in some patients, as has low-energy femoral shaft and subtrochanteric fractures.
Merck included post-marketing experiences on ONJ and thigh fractures. Under its Adverse Reactions heading, the Fosamax Plus D label now includes post-marketing experience showing localized osteonecrosis of the jaw, generally associated with tooth extraction and/or local infection with delayed healing, has been reported in some patients, as has low-energy femoral shaft and subtrochanteric fractures.Prior to the new labelling, Merck had removed from the Fosamax Precautions the FDA's proposed language indicating that osteonecrosis of the jaw had occurred in patients taking oral bisphosphonates, such as Fosamax.
The updated labeling does not include studies by the Cornell University Medical School (above).
 In July 2005 Merck added a warning on page 13 of a 22-page document it distributed to pharmacies.
In July 2005 Merck added a warning on page 13 of a 22-page document it distributed to pharmacies.The Merck website stated the following: [Fosamax] may cause jaw-bone problems in some people. Jaw-bone problems may include infection and delayed healing after teeth are pulled…improvement in bone density may be observed as early as 3 months after you start taking Fosamax, even though you won't see or feel a difference and that for fosamax to continue to work, you need to keep taking it.
The FDA ordered the makers of Fosamax and other bisphosphonates to list osteonecrosis in its precautions on package inserts. However, a lawsuit filed in a US District Court alleged that Merck deliberately concealed the drug's adverse side effects from doctors and patients and that Merck knew Fosamax could cause ONJ but still neglected to comply with the FDA's request to add a Fosamax warning to all drug labeling.
Fosamax Eye Problems
Fosamax Lawsuits
 Hundreds of individual lawsuits—and at least two class action lawsuits--have been filed against Merck & Co. relating to Fosamax and its association with osteonecrosis of the jaw—a painful and often disfiguring disorder without a cure.
Hundreds of individual lawsuits—and at least two class action lawsuits--have been filed against Merck & Co. relating to Fosamax and its association with osteonecrosis of the jaw—a painful and often disfiguring disorder without a cure.In April 2012, Merck announced it had won its fifth of six lawsuits concerning Fosamax. According to the drugmaker, a jury found that the plaintiff had medical conditions that increased the likelihood she would develop jaw problems after dental surgery, dismissing her claims that Fosamax caused her jaw problems.
2006: In April 2006, a class action suit was filed in U.S. District Court at Fort Meyers, Florida against Merck, alleging that Fosamax caused, in some cases, osteonecrosis of the jaw.
2007: A class action was filed against Merck Frosst Canada in 2007, claiming the company failed to adequately warn that one of its medications had been associated with a higher risk of developing decay of the jaw bone. The class action also named Merck & Co., the New Jersey parent company of Merck Frosst.
Merck said it had put aside $48 million to set up a defense fund for cases alleging Fosamax causes osteonecrosis, or dead jaw. The bulk of cases were filed in federal court. By May 2008 Merck faced about 100 new lawsuits claiming the company failed to warn that Fosamax may cause an irreversible jaw-rot.
2008: In July 2008 The United States District Court for the Southern District of New York remanded several cases to state courts in the States of California, Florida, and Illinois. The District Court also taxed costs and fees against Merck for the removal of the cases to federal court.
2010: The federal District courthouse in Manhattan scheduled the Boles v Merck retrial for June 7, 2010. Shirley Boles' lawyers intend to call a Merck witness to establish that by 2007, Merck had been clearly told by FDA to revise the Fosamax labeling, and to bring it into line with the widely-accepted (WHO and FDA) definitions of what constitutes osteoporosis.
In January 29, 2010 a Manhattan federal judge refused to dismiss a lawsuit alleging that Merck's Fosamax caused jaw damage to an Indiana woman during the nearly eight years she took the pill.
These cases all claim that Merck failed to adequately research Fosamax and warn about the risk of jaw problems. There are approximately 900 lawsuits currently pending against Merck & Co.
Despite Merck's claims of safety, more than 2,400 people have experienced jaw death from taking Fosamax, including cases where the jawbone rots and dies. Anyone who has taken Fosamax and experienced adverse side effects, especially relating to osteonecrosis of the jaw or femur fractures, may be eligible to become involved in litigation against Merck & Co.
According to data from IMS Heatlh, in 2008 bisphosphonate sales exceeded $3.5 billion.In the same year, over 37 million prescriptions were written for the osteoporosis medications. Merck reported that, since their market introduction, over 225 million total prescriptions have been dispensed for FOSAMAX/FOSAMAX Plus D, Actonel (risedronate) and Boniva (ibandronate) in the US alone.
Fosamax Lawyer Help
If you or a loved one has suffered from ONJ or other side effects after taking Fosamax, you may qualify for damages or remedies that may be awarded in a Fosamax class action or lawsuit. Please click the link below to submit your Fosamax complaint to a Fosamax lawyer for a free case evaluation.Last updated on
FOSAMAX LEGAL ARTICLES AND INTERVIEWS
Fosamax Femur Fracture Lawsuits Revived

Generic Fosamax Injury Lawsuit to Proceed

Fosamax Femur Expiry Date: Five Years


March 24, 2017
Santa Clara, CA: About 5,000 Fosamax personal injury cases have been given renewed life this week, after the US Court of Appeals for the Third Circuit overturned an earlier dismissal of multidistrict litigation (MDL) which claims users of Merck's osteoporosis drug suffered femur fractures. READ MORE
Generic Fosamax Injury Lawsuit to Proceed

January 21, 2015
Teva Pharmaceuticals has been denied a hearing of its appeal against a lawsuit that claims the company failed to warn of the health risks linked to generic versions of the osteoporosis drug Fosamax. The appeal stems from a lawsuit brought by Olga Pikerie, who claims her fractured thighbone resulted from her prolonged use of generic Fosamax. Pikerie alleged that Teva and other generic makers hadn't brought their warnings in line with an update of the branded Fosamax's label. READ MORE
Fosamax Femur Expiry Date: Five Years

December 11, 2014
Sandra didn’t know until her second femur break that taking Fosamax for more than five years could actually do more harm than benefit. Now she needs a knee replacement, again due to Fosamax side effects. READ MORE
READ MORE Drugs/Medical Settlements and Legal News
READ MORE Personal Injury Settlements and Legal News
READ MORE Personal Injury Settlements and Legal News

READER COMMENTS
Not happy
on
I have decided to try a weightbearing vest, changing out my vitamins to OsteoMed (or something like it), walking as much as possible using a walking cane on uneven ground and eating foods that might provide a bit more calcium.
Every time I put one tablet in my mouth, I began to feel like I was poisoning myself. The possibility of losing a tooth, jaw issues, a different kind of break, migraines... I just don't know if or what I should do.
Cathy Bena
on
Annie
on
Margaret Diehl
on
Pennsylvania
on
I am so sorry to hear all of the horror stories I have read here and on many other websites regarding bisphosphonates, particularly Fosamax. I find it surprising that Fosamax was ever FDA approved. The long-term risks to both jaws AND femurs is unmistakable. The most ridiculous part of the whole matter is this:
"Merck, the manufacturer of Fosamax, is reported to have said there has been no "causal link" demonstrated between long-term use of Fosamax and fractures. Clinical studies conducted by Merck have not shown any increased risk of fracture in any part of the body."
It's just like the tobacco companies that once said there was no "causal link" between smoking cigarettes and lung cancer or heart disease.
I'm sure Merck is fully aware of the "causal link" between admitting the truth and losing a big pile of $$$!!!
May justice be swift and thorough.
Dorothy Sams
on
Susan Davis
on
Doris Cobbs
on
carolyn midgley
on
ASKED 3 X'S AND HE SAID HE WOULD LOOK AT MY EMERGANCY ROOM EXRAY! RETURNED AND ORDERED AN XRAY! IT WAS FRACTURED THROUGH WAITING TO BREAK! HE SAID HE COUN'T PUT ME BACK IN SURGERY FOR 3 WKS!
I HAVE RODS IN BOTH FEMURS. I WAS A VERY ACTIVE 73 YR. OLD WHEN IT HAPPENED STILL ACTIVE AT 77 IN DEC. BUT HAVE HAD HIGH BLOOD PREASURE SINCE! THEN IRREGULAR HEART BEAT AND HAIR LOSS! I NEVER HAD EITHER! IN OCT. I HAD TO HAVE A PACE MAKER!
I FEEL I NEED A LAW SUIT AGAINST MY FORMER DR. AS NOTHING IS BEING DONE TO PROSECUTE FOR FEMURS! WHAT DO U THINK? THANK YOU
megann marten
on
ANONYMOUS
on
HAVING RODS IN ONES LEGS DUE TO FEMUR FRACTURES FROM USE OF FOSAMAX IS MORE PREVALENT THAN KNOWN.
MERCK KNEW THE PERILS OF TAKING FOSAMAX. ONE KNOWS THE BENEFITS AS WELL AS THE RISKS
Jane Tucker
on
ANTHONY MORRIS
on
One leg now shorter than the other.
Will never be the same.
Thanks to Boniva.
Lynn
on
Arman Ravery
on
Ramzia Gardizi
on
North Carolina
on
Massachusetts
on
Anonymous
on
Colorado
on
California
on
Newfoundland
on
Pennsylvania
on
Mississippi
on
New Hampshire
on
Maryland
on
Massachusetts
on
California
on
Texas
on
Constant PAIN, inability to eat well, loss of weight to point of concern.