LAWSUITS NEWS & LEGAL INFORMATION
Hip & Knee Replacements
Read our Hip and Knee Replacement FAQ
Were you looking for DePuy Canada or DePuy ASR Hip or DePuy Pinnacle or Zimmer Durom Cup or Zimmer NexGen lawsuits?
The past decade has seen an exponential increase in hip and knee replacements and with it, hip & knee replacement failure. With more and more baby boomers approaching an older age, hip and knee replacement surgery is becoming more common--as are hip and knee replacement complaints. Since the Depuy hip and knee replacement recalls in 2005, thousands of patients have filed a hip replacement lawsuit or a knee replacement lawsuit, claiming the medical devices are defective.
Zimmer NexGen Knee Lawsuit
| DePuy ASR Lawsuit | Biomet Hip
Zimmer Durom Cup Lawsuit | DePuy Pinnacle Lawsuit
Smith & Nephew Knee Implant Lawsuits | Stryker Hip Implant Lawsuit
Corin Hip Resurfacing Lawsuit | Zimmer Natural Knee & Hip Lawsuits
Zimmer Durom Cup Lawsuit | DePuy Pinnacle Lawsuit
Smith & Nephew Knee Implant Lawsuits | Stryker Hip Implant Lawsuit
Corin Hip Resurfacing Lawsuit | Zimmer Natural Knee & Hip Lawsuits
FREE HIP AND KNEE REPLACEMENT LAWSUIT EVALUATION
Send your Hip and Knee Replacement claim to a lawyer who will review your claim at NO COST or obligation.
GET LEGAL HELP NOW
GET LEGAL HELP NOW
Hip and Knee Replacements
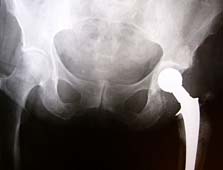 Increasingly, patients are receiving more hip and knee replacement surgeries, with about 600,000 knee replacement procedures alone performed in the US annually. Combined with a growing and worrisome number of knee replacements in younger people, both medical and legal experts predict that the amount of hip and knee failures may cost US taxpayers, insurers and employers billions of dollars in the coming years, according to The International Herald Tribune (December 2011).
Increasingly, patients are receiving more hip and knee replacement surgeries, with about 600,000 knee replacement procedures alone performed in the US annually. Combined with a growing and worrisome number of knee replacements in younger people, both medical and legal experts predict that the amount of hip and knee failures may cost US taxpayers, insurers and employers billions of dollars in the coming years, according to The International Herald Tribune (December 2011).
Because hip and knee replacements represent an entire class of products rather than simply one device or one manufacturer (see list below), the financial repercussions stemming from hip and knee implants that are alleged to be defective will likely be enormous, especially when revision surgery is taken into account.
Given the amount of hip and knee replacement surgeries performed, there has been an increase in complaints. Hip and knee implants are known to have complications, including infection and joint dislocation. Hip replacement lawyers predict that hundreds or even thousands of patients are now facing hip and knee revision surgery, or multiple revision surgeries.
One reason that so many hip and knee replacements have failed is due to manufacturers introducing their products to the market with little or no clinical evidence, according to a report in The Lancet, March 2012. When problems with hip and knee replacement devices were detected in the past, surgeons typically stopped using the faulty device and the manufacturer recalled the product. But some implants have not had a recall even though complaints have been filed--such as with Zimmer' NexGen knee and its Natural knee and hip.
A recent study reported by The International Herald Tribune, (December 2011) found that no new hip or knee implants introduced during the last five years were more durable than older devices, and 30 percent were worse.
Patients with hip and/or knee replacement failure have reported the following problem(s):
- Breakage, mainly metal breaks from constant weight-bearing stress
- Fractures typically near the artificial joint
- Loosening of the attachment between the bone and artificial device, both in cemented and un-cemented artifcial joints
- Painful stiffness and infection due to loosening of the attachment between device and bone
- Instability when the artificial joint dislocates
- Wear and tear on plastic parts
Hip Replacement Failure
Metal-on-Metal Total Hip Replacement Implant
The two types of MoM hip systems are Traditional total hip replacement implant and Total resurfacing hip implant. According to the FDA website, metal-on-metal hip implants are known to have adverse events, including infection and joint dislocation.
A report released by the National Joint Registry for England and Wales (09/11) warns that the failure rate of metal-on-metal hip devices is increasing, with the highest failure rate seen in the DePuy ASR device. According to the registry, hip replacement devices made entirely from metal had a significantly higher failure rate than those made from other materials. The failure rate was also significantly higher in women than in men. The report found that of patients who received an ASR device six years ago, approximately 30 percent had the device replaced. Failure of a hip device is not considered life-threatening, but can be debilitating.
Meanwhile, a study published in the British Medical Journal (11/11) suggested that metal-on-metal hip replacement devices are no more effective than traditional hip replacement devices and may have higher revision rates than their traditional counterparts. According to researchers, patients who receive a metal-on-metal hip replacement device have approximately double the risk of undergoing hip revision surgery.
Since 2008, the FDA has received hundreds of complaints about DePuy Orthopaedics ASR line of devices for hip surgery implants that used metal-on-metal (MoM) replacement systems, but the FDA waited until February 2011 to notify the public of its defective design.
The DePuy ASR XL modular acetabular cup and the ASR hip resurfacing system were recalled in August 2010 after hundreds of patients complained about the metal-on-metal hip implants. From August 2005 to August 2010, about 40,000 in patients in the US received the DePuy ASR and the failure rate could be as high as 13 per cent. (The generally accepted standard is no more than 5 percent of patients requiring revision surgery within five years of implantation.)
Anyone who received a hip replacement in the US after July 2003 may have received a DePuy ASR device that is defective.
DePuy ASR Infection
The design of the DePuy ASR causes the metal ball and the metal cup to slide against each other during movement, which can cause some tiny metal particles to wear off of the device and enter into the space around the implant. Metal ions from the implant may enter into the bloodstream, which may cause severe medical problems. Some patients with metal-on-metal hip implants developed a reaction to these ions, which could affect the nervous system, heart and thyroid gland. Metal debris in the bloodstream can lead to cobalt poisoning or toxicity.
DePuy ASR Slippage and/or Joint Dislocation
Metal particles around some implants can also cause damage to bone and/or tissue surrounding the implant and joint, which is called "adverse local tissue reaction (ALTR)" or an "adverse reaction to metal debris (ARMD). This reaction may cause pain and/or the implant to loosen.
On July 22, 2008, the Zimmer Durom Acetabular Component ("Durom Cup") was recalled because the instructions for use/surgical technique instructions were inadequate. Further information about the recall can be found on Zimmer's letter to surgeon.
Since it was introduced to the US market in 2006, the Durom Cup has been used for total hip replacement in more than 12,000 patients, but many individuals have reported severe pain and the need for additional hip surgeries after having the Zimmer Durom Cup hip replacement implanted. According to the manufacturer, doctors reported post-hip surgery problems "requiring revision" 5.7 percent of the time. Some patients have also experienced problems with the Zimmer NexGen CR-Flex knee replacement component.
The DePuy Pinnacle is a metal-on-metal hip implant with an unreasonably high failure rate. As of December 2010, over 500 complaints had been received by the FDA Adverse Event Reporting System from people who had experienced adverse effects from the Pinnacle system. Reports allege that the stem is loosening and slipping out of the cup and a number of patients are requiring revision surgery.
According to the FDA MAUDE Adverse Event Report, DePuy is currently investigating loss of osseointegration with the DePuy Pinnacle device.
Similar to the ASR XL Acetabular and ASR Hip Resurfacing Systems, the DePuy Pinnacle Acetabular Cup System was fast-tracked through the FDA's medical device approval process because it was similar to already-approved devices. However, unlike the other hip systems, the DePuy Pinnacle Acetabular Cup System has not yetbeen recalled. Regardless, DePuy Pinnacle lawsuits have been filed (see below).
In January 2008, Stryker Corp. recalled two hip implant products in their Trident stock. Both products are replacement cups: the Trident Acetabular PSL Cup, and the Trident Hemispherical Cup.
People who had the implants reported difficulties with the products since 2005, including severe and intense pain, trouble walking, joints that squeak, broken joint implants, and poor fit of implants leading to bone breaks.
Corin Hip Resurfacing
Hip resurfacing was introduced in the late 1990s as an alternative to the more conventional total hip replacement. It is mainly designed for middle-aged patients who are physically active and expected to outlive the l 15-20 year lifespan of a full hip replacement. However, a British study by The Royal College of Surgeons (September 2008) found about 4 percent of the 2,360 women who underwent resurfacing in England had to have a second operation to repair the same hip within three years. Those women who received traditional hip replacements had a re-operation rate of less than 1.6 percent.
US Research (Clinical Orthopaedics and Related Research, January 2009) found that most complications with hip resurfacing occurred in women of all ages and men over the age of 55. Resurfacing in the US is expected to increase, despite the risks.
Biomet Hip Replacement
Biomet has recalled three of its following products:
- 2001: Zirconia ceramic femoral heads manufactured by St. Gobain Desmarquest because they were fracturing at a higher rate than expected
- 2007: Tibial Bearing ARCOM UHMWPE due to improper laser etching for size.
- 2008: Modular Microplasty Cup Insterter because the weld at the lock location could break during impaction.
Knee Replacement Failure
Zimmer introduced two versions of the NexGen CR-Flex Fixed Bearing Knee in 2003: a cemented version (that uses an adhesive to connect the thigh bone to the device) and a cementless version (which bonds by natural bone ingrowth). Both artificial knees are supposed to last at least 15 years, but a number of orthopedic surgeons, including former consultants for Zimmer, believe the device is defective. Since 2006, reports from patients have shown higher-than-normal rates of loosening and failure in the uncemented CR-Flex Porous Femoral component. To date, there has not been a Zimmer NexGen knee recall, but product liability attorneys and personal injury attorneys are evaluating Zimmer knee claims for patients who have experienced failure with the Zimmer NexGen CR-Flex Porous Femoral component.
More than 26,000 of Zimmer' "Gender Solutions" Natural-Knee Flex System, Prolong Articular Surface, knee implants were recalled in 2009 and 2010. Although Zimmer said the devices were recalled because the surgeon may have difficulty inserting parts of the device, experienced hip and knee attorneys believe the device could be defective.
In 2012 Zimmer' Natural-Knee II Durasul Patella product was issued a Class II recall for its knee replacement component due to reported device failures.
Oxinium Pro-Fix II and the Oxinium Genesis II
In August 2003, the London-based medical device manufacturer Smith & Nephew recalled of two of their knee replacement products--the Oxinium Pro-Fix II and the Oxinium Genesis II. Some of these "cementless" knee replacements failed to bond properly, which greatly increased the possibility of infection, as well as joint, muscular and neurological damage in addition to further painful rehabilitation and revision surgery.
Other Hip and Knee Replacement Issues
Some Hip and Knee Implant failure problems can be traced back to several companies' sterilization practices. Howmedica, as well as several other companies, used a technique known as gamma irradiation in air to sterilize the devices (hip and knee prostheses) made of high molecular weight polyethylene, which is like a pliable plastic. The sterilization process caused the polyethylene part of the devices, once implanted in the body, to break up into small pieces that lodge into the patient's hip or knee joint. In the body's fight to rid itself of those pieces, the immune system also turns on the patient's healthy bone, causing it to decay, a condition known as osteolysis. These patients inevitably need a second surgery to replace the device long before expected.
Oxidation
Oxidation, which is a time-dependent byproduct of gamma sterilization in air, has an adverse effect on the material properties and wear resistance of polyethylene. Oxidation occurs when oxygen combines with free radicals in the. The oxidation can occur during irradiation, or it can occur over time as oxygen diffuses into the polyethylene and combines with residual free radicals.
Recognizing the adverse effects of oxidation, some manufacturers have abandoned gamma sterilization and began using ethylene oxide (EtO) or gas plasma to sterilize the components, thereby avoiding immediate and long-term oxidation. However, particularly in the absence of oxygen , the free radicals generated by irradiation can combine to each other, forming "crosslinks" between two carbon atoms on adjacent polyethylene molecules.
When packaging/sterilizing in a low-oxygen environment (e.g., partial vacuum, inert gas, or with an oxygen scavenger), the degree of oxidation that will occur is reduced. In order to gain the benefit of the crosslinking, some manufacturers continue to sterilize their polyethylene components with gamma radiation.
Defective Hip Implant Symptoms
The following symptoms that could occur after your hip implant surgery may indicate that your artificial knee or hip is not working as it should, and may require revision surgery:
- Pain in the groin, hip or leg
- Swelling at or near the hip joint
- A limp or change in walking ability
- Heart (chest pain, shortness of breath)
- Nerves (numbness, weakness, change in vision or hearing)
- Thyroid (fatigue, feeling cold, weight gain)
- Blood tests, including checking levels of metal ions in the blood
- Special imaging tests
- Joint aspiration (using a needle to remove fluid from around the joint )
Defective implants require removal and revision (also known as replacement) surgery at significant expense. Your orthopedic surgeon may recommend revision surgery for a number of reasons, including infection, dislocation, and device fracture. Revision surgery may be advised if you develop evidence of local or systemic reactions to the metal from your hip implant.
Researchers believe that data on knee and hip replacement surgery is mostly limited to revision surgery, which means the number of complaints from hip and knee replacement patients could be the tip of the iceberg. Many patients suffer pain and disability without undergoing revision surgery.
Complications from revision surgery include:
- Infection
- Thrombophlebitis (blood clots in the legs)
- Myositis ossificans (calcium deposits in soft tissue around the knee joint, causing inflammation of muscles where they meet the bone)
- Loosening where a metal component or cement meets the bone
- Incision complications
- Bone fractures during surgery
- Dislocation of the new prothesis
- A difference in leg length because of the artificial knee
- More or faster loss of bone tissue
Hip and Knee Replacement Lawsuits
The following manufacturers have lawsuits filed against them for faulty hip and knee implants:
- Biomet
- Depuy
- Howmedica Osteonics Corp.
- Howmedica, Inc.
- Osteonics Corp.
- Zimmer
According to Medical Industry Week, on February 18, 2011 a personal injury lawsuit was filed against Johnson & Johnson's DePuy Orthopaedics, alleging its DePuy ASR XL acetabular system is defective. The lawsuit has been filed in a Federal Court in San Francisco, CA.
One DePuy Pinnacle lawsuit, which was filed in November 2010 in the U.S. District Court for the Central District of California, claims that the plaintiff has been suffering from an abnormal gait, nerve pain and other problems since receiving the Pinnacle device in 2004. Another suit, filed in December 2010, in the U.S. District Court for the Western District of Washington, alleges the plaintiff has undergone six surgeries as a result of metal poisoning he developed after receiving a Pinnacle hip implant in 2007.
Hip & Knee Replacement Legal Help
If you feel you qualify for damages or remedies that might be awarded in a possible Hip & Knee Replacement lawsuit, please click the link below to submit your complaint to a lawyer who will review your claim at no cost or obligation.Last updated on
HIP AND KNEE REPLACEMENT LEGAL ARTICLES AND INTERVIEWS
Tracking Systems in the U.K Key to Care for Hip Implant Patients, But Non-Existent in the U.S.

DePuy Hip’s Million Dollar Surgeons “Taking Share of Business”

Stryker Defective Hip Implant Lawsuit Plaintiff Survey Deemed ‘Intrusive’


January 26, 2018
Santa Cruz, CA:The study, published in the Bone & Joint Journal, finds that the rate of metal-on-metal (MoM) hip implant patients undergoing revision has increased over time. Researchers at the Universities of Oxford and Bristol have suggested the observed differences are in part due to increased patient surveillance. In the U.S., patient surveillance is pretty much left up to the patient. READ MORE
DePuy Hip’s Million Dollar Surgeons “Taking Share of Business”

December 24, 2017
Dallas, TX: In a recent hip replacement trial, plaintiffs’ lawyer showed to the jury records whereby DePuy paid its Pinnacle hip "design surgeons" more than $184 million in royalties that he argued were kickbacks. And jurors watched a video of a 2008 DePuy sales conference themed “Taking Share of Business”. To the lyrics of “Takin’ Care of Business” by the rock group Bachman-Turner Overdrive, DePuy’s former top marketer leads a parade with costumed alligators, a bloodied man with a hatchet and a giant metal hip implant. The plaintiffs won. READ MORE
Stryker Defective Hip Implant Lawsuit Plaintiff Survey Deemed ‘Intrusive’

November 26, 2017
Boston, MA: With the date for the first bellwether hip replacement implant failure trial involving failed Stryker femoral heads now established for September, 2019 attention has shifted to a compelling questionnaire the Howmedica defense team has issued to plaintiffs as part of the deposition process. READ MORE
MORE HIP AND KNEE REPLACEMENT LEGAL NEWS
- Hip Replacement led to Sleep Deprivation and Oxycodone
- Depuy Attune Knee Victim “Pissed off that there’s No Way to Fix this Defect Without Litigation”
- Three Strikes after Depuy Synthes Attune Knee, First Lawsuit is Filed
- Coming Unglued – DePuy Synthes Attune Buckles at the Knees
READ MORE Defective Knee Implant Settlements and Legal News
READ MORE Defective Products Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News
READ MORE Defective Products Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News

READER COMMENTS
Sue L. Presley
on
. Didn’t know why I hurt. I went to another Dr. He said it needed to be replaced. I was scared to have revision thinking it might get better. It only got worse. Had to have Revision 2021. but am still having terrible pain when I stand or walk and if I lie wrong. Now I have terrible hip, and leg pain. Surgeon made X-Rays and said he thought it was my spine. Did MRI and it showed a little Spinal Stenosis. In unbearable pain .Waiting to see Spinal Doctor
Nina Simmerman
on
william Muephy
on
Mark Hagstrom
on
Robbie Riley
on
Frustrated!!!
Oscar frisch
on
Patricia
on
Now it felt like water running down my leg.My big toe,felt
Like pin in it.And my knee get water on it.The DR draw the water off.
CC Lence
on
Utah.
Deborah Stone
on
Yolanda Porter
on
Terry R Horton
on
Mary Windham-Durrah
on
Cassandra Segoviano
on
Sue Holzkamp
on
Kentucky
on
Of course when I got there I never saw my doctor again but was seen by another who said he didnt know what had happened but I would need another surgery to replace the joint.
My wife has had to give up her plans for a federal job in order to care for me as I am having difficulties and will need a new surgery. We are seeking damages for malpractice as well as pain, suffering and loss of wages.
Rickey
on
Rose Ruiz
on
Otis Welch
on
Georgia
on
Illinois
on
Pennsylvania
on
Colorado
on
Anonymous
on
Utah
on
Anonymous
on
Florida
on
Alabama
on