LAWSUITS NEWS & LEGAL INFORMATION
Paxil Birth Defects Lawyers
Read our Paxil FAQ
Recent studies have found that women who take Paxil during certain times in their pregnancy are more likely to give birth to babies experiencing Paxil side effects than women who take other antidepressants or women who do not take any antidepressants during pregnancy. These Paxil side effects can be life-threatening and include serious health problems such as Paxil heart defects. Now, some women are investigating filing their own Paxil lawsuit, alleging the company did not properly warn them about the risks associated with taking the antidepressant during pregnancy.
Last updated on
En Español PAXIL DEFECTOS DE NACIMIENTO
FREE PAXIL LAWSUIT EVALUATION
Send your Paxil claim to a lawyer who will review your claim at NO COST or obligation.
GET LEGAL HELP NOW
GET LEGAL HELP NOW
FDA Paxil Warning
The FDA reviewed two studies which found that women who took Paxil in the first three months of pregnancy were 1-1/2 to two times more likely to give birth to a child with a heart defect than women who took other antidepressants or pregnant women overall.
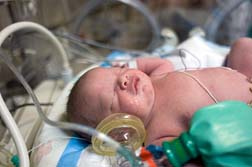 In December 2005, The Food and Drug Administration (FDA) warned pregnant women and their doctors of an increased risk of heart defects in newborns when taking the antidepressant Paxil (which goes by the generic name paroxetine). The FDA reviewed two studies which found that women who took Paxil in the first three months of pregnancy were 1 1/2 to two times more likely to give birth to a child with a heart defect than women who took other antidepressants or pregnant women overall.
In December 2005, The Food and Drug Administration (FDA) warned pregnant women and their doctors of an increased risk of heart defects in newborns when taking the antidepressant Paxil (which goes by the generic name paroxetine). The FDA reviewed two studies which found that women who took Paxil in the first three months of pregnancy were 1 1/2 to two times more likely to give birth to a child with a heart defect than women who took other antidepressants or pregnant women overall.
Along with the warning, the FDA placed the drug into its second-highest category for risk of birth defects, advising patients that "this drug should usually not be taken during pregnancy." In a later warning, the FDA said that Paxil "should generally not be initiated in women who are in their first trimester of pregnancy or in women who plan to become pregnant in the near future."
In the studies cited by the FDA, the risk of heart defects is about 1 percent overall and rose to 1.5 to 2 percent in infants born to women taking Paxil. As a consequence of these studies, GlaxoSmithKline and the FDA reclassified Paxil as a "Category D" drug for pregnant women. The classification means that studies in pregnant women have shown that the drug poses a risk to the fetus.
Researchers have been concerned about selective serotonin reuptake inhibitors (SSRIs)—Paxil is in this category—on fetuses for some time, but the FDA's announcement now indicates significant risk, particularly since a large number of pregnant women (estimated as high as 20 percent) suffer from depression and are readily prescribed antidepressants, including Paxil.
In December 2011, the FDA announced that studies gave conflicting results concerning the use of SSRIs while pregnant. Although two studies reportedly suggested a link between the use of SSRIs while pregnant and the development of PPHN, three studies did not support such a finding, making no clear link between SSRIs and PPHN. The FDA has now advised that women who are taking antidepressants while pregnant not stop.
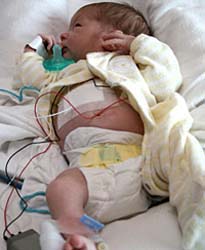 A study from Sweden found that birth defects were twice as common among Paxil users as among women taking other antidepressants or none at all, and that most birth defects involve holes and malformations in the chambers of the heart. The defects often heal on their own, but more severe cases must be surgically repaired.
A study from Sweden found that birth defects were twice as common among Paxil users as among women taking other antidepressants or none at all, and that most birth defects involve holes and malformations in the chambers of the heart. The defects often heal on their own, but more severe cases must be surgically repaired.
Since Paxil was approved in 1993, it has become one of the world's most popular antidepressants, taken by tens of millions of patients. According to GlaxoSmithKline, about 25 percent of Paxil users are women of childbearing age, between 18 and 45.
 In December 2005, The Food and Drug Administration (FDA) warned pregnant women and their doctors of an increased risk of heart defects in newborns when taking the antidepressant Paxil (which goes by the generic name paroxetine). The FDA reviewed two studies which found that women who took Paxil in the first three months of pregnancy were 1 1/2 to two times more likely to give birth to a child with a heart defect than women who took other antidepressants or pregnant women overall.
In December 2005, The Food and Drug Administration (FDA) warned pregnant women and their doctors of an increased risk of heart defects in newborns when taking the antidepressant Paxil (which goes by the generic name paroxetine). The FDA reviewed two studies which found that women who took Paxil in the first three months of pregnancy were 1 1/2 to two times more likely to give birth to a child with a heart defect than women who took other antidepressants or pregnant women overall.Along with the warning, the FDA placed the drug into its second-highest category for risk of birth defects, advising patients that "this drug should usually not be taken during pregnancy." In a later warning, the FDA said that Paxil "should generally not be initiated in women who are in their first trimester of pregnancy or in women who plan to become pregnant in the near future."
In the studies cited by the FDA, the risk of heart defects is about 1 percent overall and rose to 1.5 to 2 percent in infants born to women taking Paxil. As a consequence of these studies, GlaxoSmithKline and the FDA reclassified Paxil as a "Category D" drug for pregnant women. The classification means that studies in pregnant women have shown that the drug poses a risk to the fetus.
Researchers have been concerned about selective serotonin reuptake inhibitors (SSRIs)—Paxil is in this category—on fetuses for some time, but the FDA's announcement now indicates significant risk, particularly since a large number of pregnant women (estimated as high as 20 percent) suffer from depression and are readily prescribed antidepressants, including Paxil.
In December 2011, the FDA announced that studies gave conflicting results concerning the use of SSRIs while pregnant. Although two studies reportedly suggested a link between the use of SSRIs while pregnant and the development of PPHN, three studies did not support such a finding, making no clear link between SSRIs and PPHN. The FDA has now advised that women who are taking antidepressants while pregnant not stop.
Paxil Study
 A study from Sweden found that birth defects were twice as common among Paxil users as among women taking other antidepressants or none at all, and that most birth defects involve holes and malformations in the chambers of the heart. The defects often heal on their own, but more severe cases must be surgically repaired.
A study from Sweden found that birth defects were twice as common among Paxil users as among women taking other antidepressants or none at all, and that most birth defects involve holes and malformations in the chambers of the heart. The defects often heal on their own, but more severe cases must be surgically repaired.Since Paxil was approved in 1993, it has become one of the world's most popular antidepressants, taken by tens of millions of patients. According to GlaxoSmithKline, about 25 percent of Paxil users are women of childbearing age, between 18 and 45.
Paxil Lawsuit
Women who took Paxil during the first trimester (the first three months of pregnancy) on or before December 2005 and whose babies were born with heart defects (such as a hole in the atrial or ventral septum), persistent pulmonary hypertension of the newborn (PPHN), Omphalocele (an abdominal birth defect), Hydrocephalus (water on the brain) and/or Craniosynostosis (misshapen head) could be eligible to file a Paxil birth defects lawsuit. Women whose babies were born with heart murmurs after Paxil use during the first trimester should also contact an attorney to discuss their legal options.
In October, 2009, a jury awarded a family $2.5 million in a Paxil lawsuit filed against GlaxoSmithKline that alleged Paxil was responsible for a young boy's birth defects. The lawsuit claimed that Lyam Kilker, now three years old, suffered from Paxil birth defects, including two holes in his heart, which required surgery. Furthermore, the lawsuit argued that Lyam will require further surgery as he grows and that his heart defects were caused by exposure to Paxil prior to Lyam's birth.
GlaxoSmithKline has said it will appeal the decision. Meanwhile the company reportedly settled around 800 Paxil birth defects lawsuits for approximately $1 billion in July 2010.
In October, 2009, a jury awarded a family $2.5 million in a Paxil lawsuit filed against GlaxoSmithKline that alleged Paxil was responsible for a young boy's birth defects. The lawsuit claimed that Lyam Kilker, now three years old, suffered from Paxil birth defects, including two holes in his heart, which required surgery. Furthermore, the lawsuit argued that Lyam will require further surgery as he grows and that his heart defects were caused by exposure to Paxil prior to Lyam's birth.
GlaxoSmithKline has said it will appeal the decision. Meanwhile the company reportedly settled around 800 Paxil birth defects lawsuits for approximately $1 billion in July 2010.
Paxil Heart Defect Legal Help
If your baby was born with heart defects, and the mother took Paxil while pregnant, a lawyer may be able to help you. Please click the link below to send your Paxil birth defects complaint to an attorney who will evaluate your claim for free.Last updated on
PAXIL LEGAL ARTICLES AND INTERVIEWS
GSK to Pay $3 Billion to Settle Criminal Charges on Avandia, Paxil and Wellbutrin

Paxil Side Effects Can Include Double Risk of Pre-Term Labor

Study Questions Paxil Side Effects


July 2, 2012
In what is being touted as the largest case of healthcare fraud in US history, GlaxoSmithKline Plc (GSK) is pleading guilty to misdemeanor criminal charges and will pay $3 billion in settlement of those charges relating to its drugs Paxil, Wellbutrin, and Avandia. READ MORE
Paxil Side Effects Can Include Double Risk of Pre-Term Labor

June 15, 2012
On the surface it's an alarming finding, and while other factors may be at play the concern remains that for each SSRI prescription an expectant mom fills during the second trimester of her pregnancy, her odds of going into premature labor can double. Such is this latest concern over Paxil side effects—a popular antidepressant in the class identified as selective serotonin reuptake inhibitors (SSRI). READ MORE
Study Questions Paxil Side Effects

June 4, 2012
Yet another study has been conducted that suggests infants may be susceptible to Paxil side effects and side effects from other, similar medications. Although the study did not examine the risk of Paxil heart defects, it did study what effect maternal use of an SSRI had on a newborn infant. The study found that there is some risk of Paxil side effects, and other antidepressant side effects in infants exposed to the medication prior to birth. READ MORE
PAXIL SETTLEMENTS
- GlaxoSmithKline Pays $750 Million Over Defective Drugs
- GlaxoSmithKline $64 million class action settlement over Paxil promotion.
- GlaxoSmithKline Class action settlement awards more money to pediatric Paxil plaintiffs.
READ MORE Birth Injury and Birth Defect Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News
READ MORE Drugs/Medical Settlements and Legal News

READER COMMENTS
Alabama
on
Missouri
on
Indiana
on
California
on
Virginia
on
California
on
Texas
on
Texas
on
Texas
on
California
on
West Virginia
on
California
on